Potential role for interleukin-28B genotype in treatment decision-making in recent hepatitis C virus infection
- PMID: 20803561
- PMCID: PMC2947598
- DOI: 10.1002/hep.23850
Potential role for interleukin-28B genotype in treatment decision-making in recent hepatitis C virus infection
Abstract
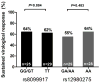
Polymorphisms in the IL28B (interleukin-28B) gene region are important in predicting outcome following therapy for chronic hepatitis C virus (HCV) infection. We evaluated the role of IL28B in spontaneous and treatment-induced clearance following recent HCV infection. The Australian Trial in Acute Hepatitis C (ATAHC) was a study of the natural history and treatment of recent HCV, as defined by positive anti-HCV antibody, preceded by either acute clinical HCV infection within the prior 12 months or seroconversion within the prior 24 months. Factors associated with spontaneous and treatment-induced HCV clearance, including variations in IL28B, were assessed. Among 163 participants, 132 were untreated (n = 52) or had persistent infection (infection duration ≥26 weeks) at treatment initiation (n = 80). Spontaneous clearance was observed in 23% (30 of 132 participants). In Cox proportional hazards analysis (without IL28B), HCV seroconversion illness with jaundice was the only factor predicting spontaneous clearance (adjusted hazards ratio = 2.86; 95% confidence interval = 1.24, 6.59; P = 0.014). Among participants with IL28B genotyping (n = 102 of 163 overall and 79 of 132 for the spontaneous clearance population), rs8099917 TT homozygosity (versus GT/GG) was the only factor independently predicting time to spontaneous clearance (adjusted hazard ratio = 3.78; 95% confidence interval = 1.04, 13.76; P = 0.044). Participants with seroconversion illness with jaundice were more frequently rs8099917 TT homozygotes than other (GG/GT) genotypes (32% versus 5%, P = 0.047). Among participants adherent to treatment and who had IL28B genotyping (n = 54), sustained virologic response was similar among TT homozygotes (18 of 29 participants, 62%) and those with GG/GT genotype (16 of 25, 64%, P = 0.884).
Conclusion: During recent HCV infection, genetic variations in IL28B region were associated with spontaneous but not treatment-induced clearance. Early therapeutic intervention could be recommended for individuals with unfavorable IL28B genotypes.
Figures




Comment in
-
Interferon induced protein 10 remains a useful biomarker of treatment failure in patients stratified for the interleukin-28B rs12979860 haplotype.Hepatology. 2011 Apr;53(4):1410-1. doi: 10.1002/hep.24055. Hepatology. 2011. PMID: 21480356 No abstract available.
References
-
- Micallef JM, Kaldor JM, Dore GJ. Spontaneous viral clearance following acute hepatitis C infection: a systematic review of longitudinal studies. J Viral Hepat. 2006;13:34–41. - PubMed
-
- Grebely J, Matthews GV, Petoumenos K, Dore GJ. Spontaneous clearance and the beneficial impact of treatment on clearance during recent hepatitis C virus infection. J Viral Hepat. 2009 - PubMed
-
- Kamal SM, Fouly AE, Kamel RR, Hockenjos B, Al Tawil A, Khalifa KE, He Q, et al. Peginterferon alfa-2b therapy in acute hepatitis C: impact of onset of therapy on sustained virologic response. Gastroenterology. 2006;130:632–638. - PubMed
-
- Wang CC, Krantz E, Klarquist J, Krows M, McBride L, Scott EP, Shaw-Stiffel T, et al. Acute hepatitis C in a contemporary US cohort: modes of acquisition and factors influencing viral clearance. J Infect Dis. 2007;196:1474–1482. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous
