Expression, functional, and structural analysis of proteins critical for otoconia development
- PMID: 20803598
- PMCID: PMC3064886
- DOI: 10.1002/dvdy.22405
Expression, functional, and structural analysis of proteins critical for otoconia development
Erratum in
- Dev Dyn. 2011 Feb;240(2):457
Abstract
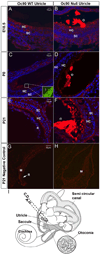
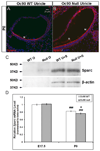
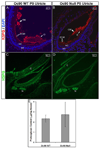
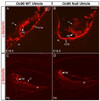
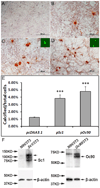
Otoconia, developed during late gestation and perinatal stages, couple mechanic force to the sensory hair cells in the vestibule for motion detection and bodily balance. In the present work, we have investigated whether compensatory deposition of another protein(s) may have taken place to partially alleviate the detrimental effects of Oc90 deletion by analyzing a comprehensive list of plausible candidates, and have found a drastic increase in the deposition of Sparc-like 1 (aka Sc1 or hevin) in Oc90 null versus wt otoconia. We show that such up-regulation is specific to Sc1, and that stable transfection of Oc90 and Sc1 full-length expression constructs in NIH/3T3 cells indeed promotes matrix calcification. Analysis and modeling of Oc90 and Sc1 protein structures show common features that may be critical requirements for the otoconial matrix backbone protein. Such information will serve as the foundation for future regenerative purposes.
Figures



References
-
- Balsamo G, Avallone B, Del GF, Trapani S, Marmo F. Calcification processes in the chick otoconia and calcium binding proteins: patterns of tetracycline incorporation and calbindin-D28K distribution. Hear Res. 2000;148:1–8. - PubMed
-
- Banfi B, Malgrange B, Knisz J, Steger K, Dubois-Dauphin M, Krause KH. NOX3, a superoxide-generating NADPH oxidase of the inner ear. J Biol Chem. 2004;279:46065–46072. - PubMed
-
- Boanini E, Torricelli P, Gazzano M, Giardino R, Bigi A. Nanocomposites of hydroxyapatite with aspartic acid and glutamic acid and their interaction with osteoblast-like cells. Biomaterials. 2006;27:4428–4433. - PubMed
-
- Bolander ME, Young MF, Fisher LW, Yamada Y, Termine JD. Osteonectin cDNA sequence reveals potential binding regions for calcium and hydroxyapatite and shows homologies with both a basement membrane protein (SPARC) and a serine proteinase inhibitor (ovomucoid) Proc Natl Acad Sci U S A. 1988;85:2919–2923. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

