Nitrile-containing pharmaceuticals: efficacious roles of the nitrile pharmacophore
- PMID: 20804202
- PMCID: PMC2988972
- DOI: 10.1021/jm100762r
Nitrile-containing pharmaceuticals: efficacious roles of the nitrile pharmacophore
Figures







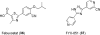


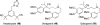
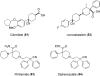
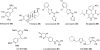




References
-
- Murphy ST, Case HL, Ellsworth E, Hagen S, Huband M, Joannides T, Limberakis C, Marotti KR, Ottolini AM, Rauckhorst M, Starr J, Stier M, Taylor C, Zhu T, Blaser A, Denny WA, Lu GL, Smaill JB, Rivault F. The synthesis and biological evaluation of novel series of nitrile-containing fluoroquinolones as antibacterial agents. Bioorg. Med. Chem. Lett. 2007;17:2150–2155. - PubMed
- Markus B, Kwon C-H. In vitro Metabolism of Aromatic Nitriles. J. Pharm. Sci-US. 1994;83:1729–1734. - PubMed
-
- MacFaul PA, Morley AD, Crawford JJ. A simple in vitro assay for assessing the reactivity of nitrile containing compounds. Bioorg. Med. Chem. Lett. 2009;19:1136–1138. - PubMed
-
- Oballa RM, Truchon J-F, Bayly CI, Chauret N, Day S, Crane S, Berthelette C. A generally applicable method for assessing the electrophilicity and reactivity of diverse nitrile-containing compounds. Bioorg. Med. Chem. Lett. 2007;17:998–1002. - PubMed
-
-
For an example encountered during the development of Cathepsin Ka and Sb analogs see: Boyd MJ, Crane SN, Robichaud J, Scheigetz J, Black WC, Chauret N, Wang Q, Masse F, Oballa RM. Investigation of ketone warheads as alternatives to the nitrile for preparation of potent and selective cathepsin K inhibitors. Bioorg. Med. Chem. Lett. 2009;19:675–679. Patterson AW, Wood WJL, Hornsby M, Lesley S, Spraggon G, Ellman JA. Identification of Selective, Nonpeptidic Nitrile Inhibitors of Cathepsin S Using the Substrate Activity Screening Method. J. Med. Chem. 2006;49:6298–6307.
-
-
-
For olprinone see: Kaneko K, Tadano K, Nakajima K, Sano Y, Yuzuriha T, Motoji N, Kawai N, Hasegawa S, Shigematsu A. Metabolic fate of loprinone hydrochloride. 1: Blood level, distribution, metabolism, and excretion after a single intravenous administration to rats. Yakubutsu Dotai. 1994;9:149–162. For lodoxamide see: Leong BKJ, Coombs JK, Petzold EN, Hanchar AJ. A dosimetric endotracheal nebulization technique for pulmonary metabolic disposition studies in laboratory animals. Inhalation Toxicology. 1988:37–51. For cimetidine see: Strong HA, Spino MJ. Chromatogr. 1987;422:301–308. Ziemniak JA, Wynn RJ, Aranda JV, Zarowitz BJ, Schentag JJ. The pharmacokinetics and metabolism of cimetidine in neonates. Dev. Pharmacol. Therap. 1984;7:30–38.
-
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Chemical Information

