Analysis of the actions of the novel dopamine receptor-directed compounds (S)-OSU6162 and ACR16 at the D2 dopamine receptor
- PMID: 20804495
- PMCID: PMC3000658
- DOI: 10.1111/j.1476-5381.2010.01010.x
Analysis of the actions of the novel dopamine receptor-directed compounds (S)-OSU6162 and ACR16 at the D2 dopamine receptor
Abstract
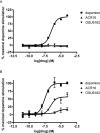
BACKGROUND AND PURPOSE; The two phenylpiperidines, OSU6162 and ACR16, have been proposed as novel drugs for the treatment of brain disorders, including schizophrenia and Huntington's disease, because of their putative dopamine stabilizing effects. Here we evaluated the activities of these compounds in a range of assays for the D(2) dopamine receptor in vitro.
Experimental approach: The affinities of these compounds for the D(2) dopamine receptor were evaluated in competition with [(3) H]spiperone and [(3) H]NPA. Agonist activity of these compounds was evaluated in terms of their ability to stimulate [(35) S]GTPγS binding.
Key results: Both compounds had low affinities for inhibition of [(3) H]spiperone binding (pK(i) vs. [(3) H]spiperone, ACR16: <5, OSU6162: 5.36). Neither compound was able to stimulate [(35) S]GTPγS binding when assayed in the presence of Na(+) ions, but if the Na(+) ions were removed, both compounds were low-affinity, partial agonists (E(max) relative to dopamine: ACR16: 10.2%, OSU6162:54.3%). Schild analysis of the effects of OSU6162 to inhibit dopamine-stimulated [(35) S]GTPγS binding indicated Schild slopes of ∼0.9, suggesting little deviation from competitive inhibition. OSU6162 was, however, able to accelerate [(3) H]NPA dissociation from D(2) dopamine receptors, indicating some allosteric effects of this compound.
Conclusions and implications: The two phenylpiperidines were low-affinity, low-efficacy partial agonists at the D(2) dopamine receptor in vitro, possibly exhibiting some allosteric effects. Comparing their in vitro and in vivo effects, the in vitro affinities were a reasonable guide to potencies in vivo. However, the lack of in vitro-in vivo correlation for agonist efficacy needs to be further addressed.
Linked articles: This article is part of a themed section on Analytical Receptor Pharmacology in Drug Discovery. To view the other articles in this section visit http://dx.doi.org/10.1111/bph.2010.161.issue-6.
© 2010 The Authors. British Journal of Pharmacology © 2010 The British Pharmacological Society.
Figures




References
-
- Carlsson A, Waters N, Holm-Waters S, Tedroff J, Nilsson M, Carlsson ML. Interactions between monoamines, glutamate, and GABA in schizophrenia: new evidence. Annu Rev Pharmacol Toxicol. 2001;41:237–260. - PubMed
-
- Castro SW, Strange PG. Differences in the ligand binding properties of the short and long versions of the D2 dopamine receptor. J Neurochem. 1993;60:372–375. - PubMed
-
- Cheng Y, Prusoff WH. Relationship between the inhibition constant (K1) and the concentration of inhibitor which causes 50 per cent inhibition (I50) of an enzymatic reaction. Biochem Pharmacol. 1973;22:3099–3108. - PubMed
-
- Ekesbo A, Torstenson R, Hartvig P, Carlsson A, Sonesson C, Waters N, et al. Effects of the substituted (S)-3-phenylpiperidine (-)-OSU6162 on PET measurements of [11C]SCH23390 and [11C]raclopride binding in primate brains. Neuropharmacology. 1999;38:331–338. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

