Characterization of sphingosine-1-phosphate lyase activity by electrospray ionization-liquid chromatography/tandem mass spectrometry quantitation of (2E)-hexadecenal
- PMID: 20804717
- PMCID: PMC2964418
- DOI: 10.1016/j.ab.2010.08.026
Characterization of sphingosine-1-phosphate lyase activity by electrospray ionization-liquid chromatography/tandem mass spectrometry quantitation of (2E)-hexadecenal
Abstract
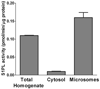
Sphingosine-1-phosphate (S1P) is a sphingolipid signaling molecule crucial for cell survival and proliferation. S1P-mediated signaling is largely controlled through its biosynthesis and degradation, and S1P lyase (S1PL) is the only known enzyme that irreversibly degrades sphingoid base-1-phosphates to phosphoethanolamine and the corresponding fatty aldehydes. S1PL-mediated degradation of S1P results in the formation of (2E)-hexadecenal, whereas hexadecanal is the product of dihydrosphingosine-1-phosphate (DHS1P) degradation. Fatty aldehydes can undergo biotransformation to fatty acids and/or alcohols, making them elusive and rendering the task of fatty aldehyde quantitation challenging. We have developed a simple, highly sensitive, and high-throughput protocol for (2E)-hexadecenal quantitation as a semicarbazone derivative by liquid chromatography-electrospray ionization-tandem mass spectrometry. The approach was applied to determining S1PL activity in vitro with the ability to use as low as 0.25μg of microsomal protein per assay. The method is also applicable to the use of total tissue homogenate as the source of S1PL. A correction for (2E)-hexadecenal disappearance due to its biotransformation during enzymatic reaction is required, especially at higher protein concentrations. The method was applied to confirm FTY720 as the inhibitor of S1PL with an IC₅₀ value of 52.4μM.
Copyright © 2010 Elsevier Inc. All rights reserved.
Figures









Similar articles
-
Method to simultaneously determine the sphingosine 1-phosphate breakdown product (2E)-hexadecenal and its fatty acid derivatives using isotope-dilution HPLC-electrospray ionization-quadrupole/time-of-flight mass spectrometry.Anal Chem. 2014 Sep 16;86(18):9065-73. doi: 10.1021/ac501677y. Epub 2014 Sep 3. Anal Chem. 2014. PMID: 25137547
-
Novel methods for the quantification of (2E)-hexadecenal by liquid chromatography with detection by either ESI QTOF tandem mass spectrometry or fluorescence measurement.Anal Chim Acta. 2012 Apr 13;722:70-9. doi: 10.1016/j.aca.2012.01.063. Epub 2012 Feb 13. Anal Chim Acta. 2012. PMID: 22444536
-
A Bioassay Using a Pentadecanal Derivative to Measure S1P Lyase Activity.Int J Mol Sci. 2021 Feb 1;22(3):1438. doi: 10.3390/ijms22031438. Int J Mol Sci. 2021. PMID: 33535437 Free PMC article.
-
Epigenetic regulation of pro-inflammatory cytokine secretion by sphingosine 1-phosphate (S1P) in acute lung injury: Role of S1P lyase.Adv Biol Regul. 2017 Jan;63:156-166. doi: 10.1016/j.jbior.2016.09.007. Epub 2016 Sep 29. Adv Biol Regul. 2017. PMID: 27720306 Free PMC article. Review.
-
Sphingosine-1-phosphate: boon and bane for the brain.Cell Physiol Biochem. 2014;34(1):148-57. doi: 10.1159/000362991. Epub 2014 Jun 16. Cell Physiol Biochem. 2014. PMID: 24977488 Review.
Cited by
-
The sphingolipid degradation product trans-2-hexadecenal induces cytoskeletal reorganization and apoptosis in a JNK-dependent manner.Cell Signal. 2011 Jul;23(7):1144-52. doi: 10.1016/j.cellsig.2011.02.009. Epub 2011 Mar 6. Cell Signal. 2011. PMID: 21385609 Free PMC article.
-
Nuclear Sphingosine-1-phosphate Lyase Generated ∆2-hexadecenal is A Regulator of HDAC Activity and Chromatin Remodeling in Lung Epithelial Cells.Cell Biochem Biophys. 2021 Sep;79(3):575-592. doi: 10.1007/s12013-021-01005-9. Epub 2021 Jun 3. Cell Biochem Biophys. 2021. PMID: 34085165 Free PMC article.
-
S1P and plasmalogen derived fatty aldehydes in cellular signaling and functions.Biochim Biophys Acta Mol Cell Biol Lipids. 2020 Jul;1865(7):158681. doi: 10.1016/j.bbalip.2020.158681. Epub 2020 Mar 12. Biochim Biophys Acta Mol Cell Biol Lipids. 2020. PMID: 32171908 Free PMC article. Review.
-
Recent Insight into the Role of Sphingosine-1-Phosphate Lyase in Neurodegeneration.Int J Mol Sci. 2023 Mar 24;24(7):6180. doi: 10.3390/ijms24076180. Int J Mol Sci. 2023. PMID: 37047151 Free PMC article. Review.
-
Copper import in Escherichia coli by the yersiniabactin metallophore system.Nat Chem Biol. 2017 Sep;13(9):1016-1021. doi: 10.1038/nchembio.2441. Epub 2017 Jul 24. Nat Chem Biol. 2017. PMID: 28759019 Free PMC article.
References
-
- Hannun YA, Obeid LM. Principles of bioactive lipid signalling: lessons from sphingolipids. Nat. Rev. Mol. Cell. Biol. 2008;9:139–150. - PubMed
-
- Schwab SR, Pereira JP, Matloubian M, Xu Y, Huang Y, Cyster JG. Lymphocyte sequestration through S1P lyase inhibition and disruption of S1P gradients. Science. 2005;309:1735–1739. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

