Multi-step oxidations catalyzed by cytochrome P450 enzymes: Processive vs. distributive kinetics and the issue of carbonyl oxidation in chemical mechanisms
- PMID: 20804723
- PMCID: PMC3010332
- DOI: 10.1016/j.abb.2010.08.017
Multi-step oxidations catalyzed by cytochrome P450 enzymes: Processive vs. distributive kinetics and the issue of carbonyl oxidation in chemical mechanisms
Abstract
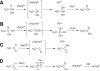
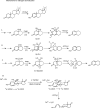
Catalysis of sequential oxidation reactions is not unusual in cytochrome P450 (P450) reactions, not only in steroid metabolism but also with many xenobiotics. One issue is how processive/distributive these reactions are, i.e., how much do the "intermediate" products dissociate. Our work with human P450s 2E1, 2A6, and 19A1 on this subject has revealed a mixture of systems, surprisingly with a more distributive mechanism with an endogenous substrate (P450 19A1) than for some xenobiotics (P450s 2E1, 2A6). One aspect of this research involves carbonyl intermediates, and the choice of catalytic mechanism is linked to the hydration state of the aldehyde. The non-enzymatic rates of hydration and dehydration of carbonyls are not rapid and whether P450s catalyze the reversible hydration is unknown. If carbonyl hydration and dehydration are slow, the mechanism may be set by the carbonyl hydration status.
Copyright © 2010 Elsevier Inc. All rights reserved.
Figures


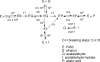

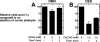
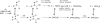

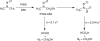
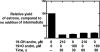


Similar articles
-
Updates on Mechanisms of Cytochrome P450 Catalysis of Complex Steroid Oxidations.Int J Mol Sci. 2024 Aug 20;25(16):9020. doi: 10.3390/ijms25169020. Int J Mol Sci. 2024. PMID: 39201706 Free PMC article. Review.
-
Common and uncommon cytochrome P450 reactions related to metabolism and chemical toxicity.Chem Res Toxicol. 2001 Jun;14(6):611-50. doi: 10.1021/tx0002583. Chem Res Toxicol. 2001. PMID: 11409933 Review.
-
Kinetic analysis of the three-step steroid aromatase reaction of human cytochrome P450 19A1.J Biol Chem. 2010 Jun 4;285(23):17734-43. doi: 10.1074/jbc.M110.123711. Epub 2010 Apr 12. J Biol Chem. 2010. PMID: 20385561 Free PMC article.
-
Studies on the mechanism of aromatase and other cytochrome P450 mediated deformylation reactions.J Steroid Biochem Mol Biol. 1993 Mar;44(4-6):367-73. doi: 10.1016/0960-0760(93)90240-w. J Steroid Biochem Mol Biol. 1993. PMID: 8476750 Review.
-
Kinetic analysis of the activation of 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone by heterologously expressed human P450 enzymes and the effect of P450-specific chemical inhibitors on this activation in human liver microsomes.Arch Biochem Biophys. 1996 Sep 1;333(1):127-38. doi: 10.1006/abbi.1996.0373. Arch Biochem Biophys. 1996. PMID: 8806763
Cited by
-
An overview of the cytochrome P450 enzymes that catalyze the same-site multistep oxidation reactions in biotechnologically relevant selected actinomycete strains.Appl Microbiol Biotechnol. 2021 Apr;105(7):2647-2661. doi: 10.1007/s00253-021-11216-y. Epub 2021 Mar 12. Appl Microbiol Biotechnol. 2021. PMID: 33710358 Review.
-
Structural and kinetic basis of steroid 17α,20-lyase activity in teleost fish cytochrome P450 17A1 and its absence in cytochrome P450 17A2.J Biol Chem. 2015 Feb 6;290(6):3248-68. doi: 10.1074/jbc.M114.627265. Epub 2014 Dec 22. J Biol Chem. 2015. PMID: 25533464 Free PMC article.
-
Mechanism of the third oxidative step in the conversion of androgens to estrogens by cytochrome P450 19A1 steroid aromatase.J Am Chem Soc. 2014 Oct 22;136(42):15016-25. doi: 10.1021/ja508185d. Epub 2014 Oct 10. J Am Chem Soc. 2014. PMID: 25252141 Free PMC article.
-
Allosteric activation of cytochrome P450 3A4 by α-naphthoflavone: branch point regulation revealed by isotope dilution analysis.Biochemistry. 2011 Nov 22;50(46):10041-51. doi: 10.1021/bi2013454. Epub 2011 Oct 28. Biochemistry. 2011. PMID: 22004098 Free PMC article.
-
Oxidation of methyl and ethyl nitrosamines by cytochrome P450 2E1 and 2B1.Biochemistry. 2012 Dec 18;51(50):9995-10007. doi: 10.1021/bi301092c. Epub 2012 Dec 4. Biochemistry. 2012. PMID: 23186213 Free PMC article.
References
-
- Ortiz de Montellano PR, editor. Cytochrome P450: Structure, Mechanism, and Biochemistry. 3rd ed. Kluwer Academic/Plenum Publishers; New York: 2005.
-
- Ortiz De Montellano PR, De Voss JJ. In: Cytochrome P450: Structure, Mechanism, and Biochemistry. 3rd ed. Ortiz De Montellano PR, editor. Kluwer Academic/Plenum Publishers; New York: 2005. pp. 183–245.
-
- Guengerich FP. Chem. Res. Toxicol. 2001;14:611–650. - PubMed
-
- Guengerich FP. Chem. Res. Toxicol. 2007;21:70–83. - PubMed
-
- Guengerich FP. In: Cytochrome P450: Structure, Mechanism, and Biochemistry. 3rd ed. Ortiz de Montellano PR, editor. Kluwer Academic/Plenum Press; New York: 2005. pp. 377–530.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

