Trophic factors GDNF and BDNF improve function of retinal sheet transplants
- PMID: 20804751
- PMCID: PMC4564299
- DOI: 10.1016/j.exer.2010.08.022
Trophic factors GDNF and BDNF improve function of retinal sheet transplants
Abstract
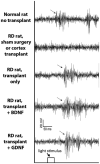
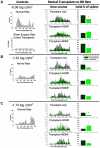
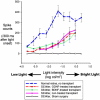
The aim of this study was to compare glial-derived neurotrophic factor (GDNF) treatment with brain-derived neurotrophic factor (BDNF) treatment of retinal transplants on restoration of visual responses in the superior colliculus (SC) of the S334ter line 3 rat model of rapid retinal degeneration (RD). RD rats (age 4-6 weeks) received subretinal transplants of intact sheets of fetal retina expressing the marker human placental alkaline phosphatase (hPAP). Experimental groups included: (1) untreated retinal sheet transplants, (2) GDNF-treated transplants, (3) BDNF-treated transplants, (4) none surgical, age-matched RD rats, (5) sham surgery RD controls, (6) progenitor cortex transplant RD controls, and (7) normal pigmented rat controls. At 2-8 months after transplantation, multi-unit visual responses were recorded from the SC using a 40 ms full-field stimulus (-5.9 to +1 log cd/m(2)) after overnight dark-adaptation. Responses were analyzed for light thresholds, spike counts, response latencies, and location within the SC. Transplants were grouped into laminated or rosetted (more disorganized) transplants based on histological analysis. Visual stimulation of control RD rats evoked no responses. In RD rats with retinal transplants, a small area of the SC corresponding to the position of the transplant in the host retina, responded to light stimulation between -4.5 and -0.08 log cd/m(2), whereas the light threshold of normal rats was at or below -5 log cd/m(2) all over the SC. Overall, responses in the SC in rats with laminated transplants had lower response thresholds and were distributed over a wider area than rats with rosetted transplants. BDNF treatment improved responses (spike counts, light thresholds and responsive areas) of rats with laminated transplants whereas GDNF treatment improved responses from rats with both laminated and rosetted (more disorganized) transplants. In conclusion, treatment of retinal transplants with GDNF and BDNF improved the restoration of visual responses in RD rats; and GDNF appears to exert greater overall restoration than BDNF.
Copyright © 2010 Elsevier Ltd. All rights reserved.
Figures





Similar articles
-
BDNF-treated retinal progenitor sheets transplanted to degenerate rats: improved restoration of visual function.Exp Eye Res. 2008 Jan;86(1):92-104. doi: 10.1016/j.exer.2007.09.012. Epub 2007 Oct 2. Exp Eye Res. 2008. PMID: 17983616 Free PMC article.
-
Structure and function of embryonic rat retinal sheet transplants.Curr Eye Res. 2007 Sep;32(9):781-9. doi: 10.1080/02713680701530597. Curr Eye Res. 2007. PMID: 17882711
-
Vision Recovery and Connectivity by Fetal Retinal Sheet Transplantation in an Immunodeficient Retinal Degenerate Rat Model.Invest Ophthalmol Vis Sci. 2017 Jan 1;58(1):614-630. doi: 10.1167/iovs.15-19028. Invest Ophthalmol Vis Sci. 2017. PMID: 28129425 Free PMC article.
-
Cell replacement and visual restoration by retinal sheet transplants.Prog Retin Eye Res. 2012 Nov;31(6):661-87. doi: 10.1016/j.preteyeres.2012.06.003. Epub 2012 Jul 5. Prog Retin Eye Res. 2012. PMID: 22771454 Free PMC article. Review.
-
Progress in retinal sheet transplantation.Prog Retin Eye Res. 2004 Sep;23(5):475-94. doi: 10.1016/j.preteyeres.2004.05.003. Prog Retin Eye Res. 2004. PMID: 15302347 Review.
Cited by
-
Retinoid receptors trigger neuritogenesis in retinal degenerations.FASEB J. 2012 Jan;26(1):81-92. doi: 10.1096/fj.11-192914. Epub 2011 Sep 22. FASEB J. 2012. PMID: 21940995 Free PMC article.
-
Computational molecular phenotyping of retinal sheet transplants to rats with retinal degeneration.Eur J Neurosci. 2012 Jun;35(11):1692-704. doi: 10.1111/j.1460-9568.2012.08078.x. Epub 2012 May 17. Eur J Neurosci. 2012. PMID: 22594836 Free PMC article.
-
Phenotypic characterization of P23H and S334ter rhodopsin transgenic rat models of inherited retinal degeneration.Exp Eye Res. 2018 Feb;167:56-90. doi: 10.1016/j.exer.2017.10.023. Epub 2017 Nov 6. Exp Eye Res. 2018. PMID: 29122605 Free PMC article.
-
Retinal Organoid Technology: Where Are We Now?Int J Mol Sci. 2021 Sep 23;22(19):10244. doi: 10.3390/ijms221910244. Int J Mol Sci. 2021. PMID: 34638582 Free PMC article. Review.
-
Sheets of human retinal progenitor transplants improve vision in rats with severe retinal degeneration.Exp Eye Res. 2018 Sep;174:13-28. doi: 10.1016/j.exer.2018.05.017. Epub 2018 May 18. Exp Eye Res. 2018. PMID: 29782826 Free PMC article.
References
-
- Aramant RB, Seiler MJ. Retinal transplantation – advantages of intact fetal sheets. Prog. Retin. Eye Res. 2002;21:57–73. - PubMed
-
- Aramant RB, Seiler MJ. Progress in retinal sheet transplantation. Prog. Retin. Eye Res. 2004;23:475–494. - PubMed
-
- Barnstable CJ, Hofstein R, Akagawa K. A marker of early amacrine cell development in rat retina. Brain Res. 1985;352:286–290. - PubMed
-
- Berardi N, Maffei L. The visual neurosciences. In: Chalupa LM, Werner JS, editors. Neurotrophins. Electrical Activity and the Development of Visual Function. Vol. 1. MIT Press; 2004.
-
- Berson EL, Rosner B, Weigel-DiFranco C, Dryja TP, Sandberg MA. Disease progression in patients with dominant retinitis pigmentosa and rhodopsin mutations. Invest. Ophthalmol. Vis. Sci. 2002;43:3027–3036. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

