The novel pyrrolidine nor-lobelane analog UKCP-110 [cis-2,5-di-(2-phenethyl)-pyrrolidine hydrochloride] inhibits VMAT2 function, methamphetamine-evoked dopamine release, and methamphetamine self-administration in rats
- PMID: 20805303
- PMCID: PMC2993560
- DOI: 10.1124/jpet.110.172742
The novel pyrrolidine nor-lobelane analog UKCP-110 [cis-2,5-di-(2-phenethyl)-pyrrolidine hydrochloride] inhibits VMAT2 function, methamphetamine-evoked dopamine release, and methamphetamine self-administration in rats
Abstract
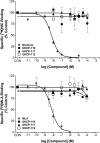
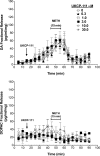
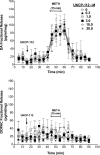
Both lobeline and lobelane attenuate methamphetamine self-administration in rats by decreasing methamphetamine-induced dopamine release via interaction with vesicular monoamine transporter-2 (VMAT2). A novel derivative of nor-lobelane, cis-2,5-di-(2-phenethyl)-pyrrolidine hydrochloride (UKCP-110), and its trans-isomers, (2R,5R)-trans-di-(2-phenethyl)-pyrrolidine hydrochloride (UKCP-111) and (2S,5S)-trans-di-(2-phenethyl)-pyrrolidine hydrochloride (UKCP-112), were evaluated for inhibition of [(3)H]dihydrotetrabenazine binding and [(3)H]dopamine uptake by using a rat synaptic vesicle preparation to assess VMAT2 interaction. Compounds were evaluated for inhibition of [(3)H]nicotine and [(3)H]methyllycaconitine binding to assess interaction with the major nicotinic receptor subtypes. In addition, compounds were evaluated for inhibition of methamphetamine-evoked endogenous dopamine release by using striatal slices. The most promising compound, UKCP-110, was evaluated for its ability to decrease methamphetamine self-administration and methamphetamine discriminative stimulus cues and for its effect on food-maintained operant responding. UKCP-110, UKCP-111, and UKCP-112 inhibited [(3)H]dihydrotetrabenazine binding (K(i) = 2.66 ± 0.37, 1.05 ± 0.10, and 3.80 ± 0.31 μM, respectively) and had high potency inhibiting [(3)H]dopamine uptake (K(i) = 0.028 ± 0.001, 0.046 ± 0.008, 0.043 ± 0.004 μM, respectively), but lacked affinity at nicotinic receptors. Although the trans-isomers did not alter methamphetamine-evoked dopamine release, UKCP-110 inhibited (IC(50) = 1.8 ± 0.2 μM; I(max) = 67.18 ± 6.11 μM) methamphetamine-evoked dopamine release. At high concentrations, UKCP-110 also increased extracellular dihydroxyphenylacetic acid. It is noteworthy that UKCP-110 decreased the number of methamphetamine self-infusions, while having no effect on food-reinforced behavior or the methamphetamine stimulus cue. Thus, UKCP-110 represents a new lead in the development of novel pharmacotherapies for the treatment of methamphetamine abuse.
Figures

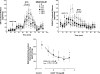



References
-
- Baumann MH, Phillips JM, Ayestas MA, Ali SF, Rice KC, Rothman RB. (2002) Preclinical evaluation of GBR12909 decanoate as a long-acting medication for methamphetamine dependence. Ann NY Acad Sci 965:92–108 - PubMed
-
- Cheng Y, Prusoff WH. (1973) Relationship between the inhibition constant (K1) and the concentration of inhibitor which causes 50 per cent inhibition (I50) of an enzymatic reaction. Biochem Pharmacol 22:3099–3108 - PubMed
-
- Decker MW, Brioni JD, Bannon AW, Arneric SP. (1995) Diversity of neuronal nicotinic acetylcholine receptors: lessons from behavior and implications for CNS therapeutics. Life Sci 56:545–570 - PubMed
-
- Dews PB. (1958) Studies on behavior. IV. Stimulant actions of methamphetamine. J Pharmacol Exp Ther 122:137–147 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

