WAVY LEAF1, an ortholog of Arabidopsis HEN1, regulates shoot development by maintaining MicroRNA and trans-acting small interfering RNA accumulation in rice
- PMID: 20805329
- PMCID: PMC2971610
- DOI: 10.1104/pp.110.160234
WAVY LEAF1, an ortholog of Arabidopsis HEN1, regulates shoot development by maintaining MicroRNA and trans-acting small interfering RNA accumulation in rice
Abstract
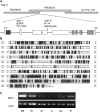
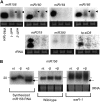
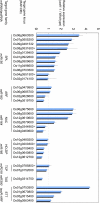
In rice (Oryza sativa), trans-acting small interfering RNA (ta-siRNA) is essential for shoot development, including shoot apical meristem (SAM) formation and leaf morphogenesis. The rice wavy leaf1 (waf1) mutant has been identified as an embryonic mutant resembling shoot organization1 (sho1) and sho2, homologs of a loss-of-function mutant of DICER-LIKE4 and a hypomorphic mutant of ARGONAUTE7, respectively, which both act in the ta-siRNA production pathway. About half of the waf1 mutants showed seedling lethality due to defects in SAM maintenance, but the rest survived to the reproductive phase and exhibited pleiotropic phenotypes in leaf morphology and floral development. Map-based cloning of WAF1 revealed that it encodes an RNA methyltransferase, a homolog of Arabidopsis (Arabidopsis thaliana) HUA ENHANCER1. The reduced accumulation of small RNAs in waf1 indicated that the stability of the small RNA was decreased. Despite the greatly reduced level of microRNAs and ta-siRNA, microarray and reverse transcription-polymerase chain reaction experiments revealed that the expression levels of their target genes were not always enhanced. A double mutant between sho and waf1 showed an enhanced SAM defect, suggesting that the amount and/or quality of ta-siRNA is crucial for SAM maintenance. Our results indicate that stabilization of small RNAs by WAF1 is indispensable for rice development, especially for SAM maintenance and leaf morphogenesis governed by the ta-siRNA pathway. In addition, the inconsistent relationship between the amount of small RNAs and the level of the target mRNA in waf1 suggest that there is a complex regulatory mechanism that modifies the effects of microRNA/ta-siRNA on the expression of the target gene.
Figures
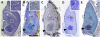




References
-
- Allen E, Xie Z, Gustafson AM, Carrington JC. (2005) MicroRNA-directed phasing during trans-acting siRNA biogenesis in plants. Cell 121: 207–221 - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
LinkOut - more resources
Full Text Sources

