Adhesion, invasion, and agglutination mediated by two trimeric autotransporters in the human uropathogen Proteus mirabilis
- PMID: 20805336
- PMCID: PMC2976323
- DOI: 10.1128/IAI.00718-10
Adhesion, invasion, and agglutination mediated by two trimeric autotransporters in the human uropathogen Proteus mirabilis
Abstract
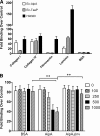
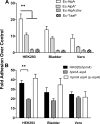
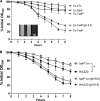
Fimbriae of the human uropathogen Proteus mirabilis are the only characterized surface proteins that contribute to its virulence by mediating adhesion and invasion of the uroepithelia. PMI2122 (AipA) and PMI2575 (TaaP) are annotated in the genome of strain HI4320 as trimeric autotransporters with "adhesin-like" and "agglutinating adhesin-like" properties, respectively. The C-terminal 62 amino acids (aa) in AipA and 76 aa in TaaP are homologous to the translocator domains of YadA from Yersinia enterocolitica and Hia from Haemophilus influenzae. Comparative protein modeling using the Hia three-dimensional structure as a template predicted that each of these domains would contain four antiparallel beta sheets and that they formed homotrimers. Recombinant AipA and TaaP were seen as ∼28 kDa and ∼78 kDa, respectively, in Escherichia coli, and each also formed high-molecular-weight homotrimers, thus supporting this model. E. coli synthesizing AipA or TaaP bound to extracellular matrix proteins with a 10- to 60-fold-higher level of affinity than the control strain. Inactivation of aipA in P. mirabilis strains significantly (P < 0.01) reduced the mutants' ability to adhere to or invade HEK293 cell monolayers, and the functions were restored upon complementation. A 51-aa-long invasin region in the AipA passenger domain was required for this function. E. coli expressing TaaP mediated autoagglutination, and a taaP mutant of P. mirabilis showed significantly (P < 0.05) more reduced aggregation than HI4320. Gly-247 in AipA and Gly-708 in TaaP were indispensable for trimerization and activity. AipA and TaaP individually offered advantages to P. mirabilis in a murine model. This is the first report characterizing trimeric autotransporters in P. mirabilis as afimbrial surface adhesins and autoagglutinins.
Figures




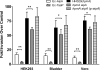
References
-
- Alamuri, P., and H. L. Mobley. 2008. A novel autotransporter of uropathogenic Proteus mirabilis is both a cytotoxin and an agglutinin. Mol. Microbiol. 68:997-1017. - PubMed
-
- Altman, E., B. A. Harrison, R. K. Latta, K. K. Lee, J. F. Kelly, and P. Thibault. 2001. Galectin-3-mediated adherence of Proteus mirabilis to Madin-Darby canine kidney cells. Biochem. Cell Biol. 79:783-788. - PubMed
-
- Barenkamp, S. J., and J. W. St. Geme III. 1996. Identification of a second family of high-molecular-weight adhesion proteins expressed by non-typable Haemophilus influenzae. Mol. Microbiol. 19:1215-1223. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

