The detrimental effects of IFN-α on vasculogenesis in lupus are mediated by repression of IL-1 pathways: potential role in atherogenesis and renal vascular rarefaction
- PMID: 20805419
- PMCID: PMC2978924
- DOI: 10.4049/jimmunol.1001782
The detrimental effects of IFN-α on vasculogenesis in lupus are mediated by repression of IL-1 pathways: potential role in atherogenesis and renal vascular rarefaction
Abstract
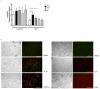
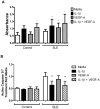
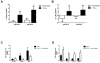
Systemic lupus erythematosus (SLE) is characterized by increased vascular risk due to premature atherosclerosis independent of traditional risk factors. We previously proposed that IFN-α plays a crucial role in premature vascular damage in SLE. IFN-α alters the balance between endothelial cell apoptosis and vascular repair mediated by endothelial progenitor cells (EPCs) and myeloid circulating angiogenic cells (CACs). In this study, we demonstrate that IFN-α promotes an antiangiogenic signature in SLE and control EPCs/CACs, characterized by transcriptional repression of IL-1α and β, IL-1R1, and vascular endothelial growth factor A, and upregulation of IL-1R antagonist and the decoy receptor IL-1R2. IL-1β promotes significant improvement in the functional capacity of lupus EPCs/CACs, therefore abrogating the deleterious effects of IFN-α. The beneficial effects from IL-1 are mediated, at least in part, by increases in EPC/CAC proliferation, by decreases in EPC/CAC apoptosis, and by preventing the skewing of CACs toward nonangiogenic pathways. IFN-α induces STAT2 and 6 phosphorylation in EPCs/CACs, and JAK inhibition abrogates the transcriptional antiangiogenic changes induced by IFN-α in these cells. Immunohistochemistry of renal biopsies from patients with lupus nephritis, but not anti-neutrophil cytoplasmic Ab-positive vasculitis, showed this pathway to be operational in vivo, with increased IL-1R antagonist, downregulation of vascular endothelial growth factor A, and glomerular and blood vessel decreased capillary density, compared with controls. Our study introduces a novel putative pathway by which type I IFNs may interfere with vascular repair in SLE through repression of IL-1-dependent pathways. This could promote atherosclerosis and loss of renal function in this disease.
Figures

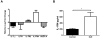

Similar articles
-
Inflammasome activation of IL-18 results in endothelial progenitor cell dysfunction in systemic lupus erythematosus.J Immunol. 2011 Dec 1;187(11):6143-56. doi: 10.4049/jimmunol.1101284. Epub 2011 Nov 4. J Immunol. 2011. PMID: 22058412 Free PMC article.
-
Interferon-alpha promotes abnormal vasculogenesis in lupus: a potential pathway for premature atherosclerosis.Blood. 2007 Oct 15;110(8):2907-15. doi: 10.1182/blood-2007-05-089086. Epub 2007 Jul 16. Blood. 2007. PMID: 17638846 Free PMC article. Clinical Trial.
-
Interleukin 10 hampers endothelial cell differentiation and enhances the effects of interferon α on lupus endothelial cell progenitors.Rheumatology (Oxford). 2015 Jun;54(6):1114-23. doi: 10.1093/rheumatology/keu431. Epub 2014 Nov 20. Rheumatology (Oxford). 2015. PMID: 25416712 Free PMC article.
-
IFN-I Mediates Dysfunction of Endothelial Progenitor Cells in Atherosclerosis of Systemic Lupus Erythematosus.Front Immunol. 2020 Nov 11;11:581385. doi: 10.3389/fimmu.2020.581385. eCollection 2020. Front Immunol. 2020. PMID: 33262760 Free PMC article. Review.
-
Accelerated atherosclerosis in systemic lupus erythematosus: role of proinflammatory cytokines and therapeutic approaches.Curr Allergy Asthma Rep. 2012 Feb;12(1):25-32. doi: 10.1007/s11882-011-0236-1. Curr Allergy Asthma Rep. 2012. PMID: 22113625 Review.
Cited by
-
Atherosclerosis in systemic lupus erythematosus.J Cardiovasc Pharmacol. 2013 Sep;62(3):255-62. doi: 10.1097/FJC.0b013e31829dd857. J Cardiovasc Pharmacol. 2013. PMID: 23792700 Free PMC article. Review.
-
Dysfunction of endothelial progenitor cells is associated with the type I IFN pathway in patients with polymyositis and dermatomyositis.Rheumatology (Oxford). 2016 Nov;55(11):1987-1992. doi: 10.1093/rheumatology/kew288. Epub 2016 Aug 7. Rheumatology (Oxford). 2016. PMID: 27498356 Free PMC article.
-
Taming lupus-a new understanding of pathogenesis is leading to clinical advances.Nat Med. 2012 Jun 6;18(6):871-82. doi: 10.1038/nm.2752. Nat Med. 2012. PMID: 22674006 Free PMC article. Review.
-
The role IL-1 in tumor-mediated angiogenesis.Front Physiol. 2014 Mar 28;5:114. doi: 10.3389/fphys.2014.00114. eCollection 2014. Front Physiol. 2014. PMID: 24734023 Free PMC article. Review.
-
Interferon-α as a biomarker to predict renal outcomes in lupus nephritis.Lupus Sci Med. 2024 Nov 28;11(2):e001347. doi: 10.1136/lupus-2024-001347. Lupus Sci Med. 2024. PMID: 39613334 Free PMC article.
References
-
- Herrmann M, Voll RE, Kalden JR. Etiopathogenesis of systemic lupus erythematosus. Immunol Today. 2000;21:424–426. - PubMed
-
- Bagavant H, Fu SM. New insights from murine lupus: disassociation of autoimmunity and end organ damage and the role of T cells. Curr Opin Rheumatol. 2005;17:523–528. - PubMed
-
- Grande JP. Mechanisms of progression of renal damage in lupus nephritis: pathogenesis of renal scarring. Lupus. 1998;7:604–610. - PubMed
-
- Kao AH, Sabatine JM, Manzi S. Update on vascular disease in systemic lupus erythematosus. Curr Opin Rheumatol. 2003;15:519–527. - PubMed
-
- Ward MM. Premature morbidity from cardiovascular and cerebrovascular diseases in women with systemic lupus erythematosus. Arthritis Rheum. 1999;42:338–346. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

