Membrane-shaping host reticulon proteins play crucial roles in viral RNA replication compartment formation and function
- PMID: 20805477
- PMCID: PMC2941330
- DOI: 10.1073/pnas.1011105107
Membrane-shaping host reticulon proteins play crucial roles in viral RNA replication compartment formation and function
Abstract
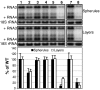
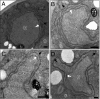
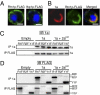
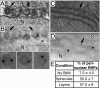
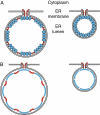
Positive-strand RNA viruses replicate their genomes on membranes with virus-induced rearrangements such as single- or double-membrane vesicles, but the mechanisms of such rearrangements, including the role of host proteins, are poorly understood. Brome mosaic virus (BMV) RNA synthesis occurs in ≈70 nm, negatively curved endoplasmic reticulum (ER) membrane invaginations induced by multifunctional BMV protein 1a. We show that BMV RNA replication is inhibited 80-90% by deleting the reticulon homology proteins (RHPs), a family of membrane-shaping proteins that normally induce and stabilize positively curved peripheral ER membrane tubules. In RHP-depleted cells, 1a localized normally to perinuclear ER membranes and recruited the BMV 2a(pol) polymerase. However, 1a failed to induce ER replication compartments or to recruit viral RNA templates. Partial RHP depletion allowed formation of functional replication vesicles but reduced their diameter by 30-50%. RHPs coimmunoprecipitated with 1a and 1a expression redirected >50% of RHPs from peripheral ER tubules to the interior of BMV-induced RNA replication compartments on perinuclear ER. Moreover, RHP-GFP fusions retained 1a interaction but shifted 1a-induced membrane rearrangements from normal vesicles to double membrane layers, a phenotype also induced by excess 1a-interacting 2a(pol). Thus, RHPs interact with 1a, are incorporated into RNA replication compartments, and are required for multiple 1a functions in replication compartment formation and function. The results suggest possible RHP roles in the bodies and necks of replication vesicles.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

