Focal Mullerian duct retention in male mice with constitutively activated beta-catenin expression in the Mullerian duct mesenchyme
- PMID: 20805501
- PMCID: PMC2941287
- DOI: 10.1073/pnas.1011606107
Focal Mullerian duct retention in male mice with constitutively activated beta-catenin expression in the Mullerian duct mesenchyme
Abstract
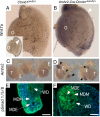
Müllerian-inhibiting substance (MIS), which is produced by fetal Sertoli cells shortly after commitment of the bipotential gonads to testicular differentiation, causes Müllerian duct (MD) regression. In the fetal female gonads, MIS is not expressed and the MDs will differentiate into the internal female reproductive tract. We have investigated whether dysregulated β-catenin activity affects MD regression by expressing a constitutively activated nuclear form of β-catenin in the MD mesenchyme. We show that constitutively activated (CA) β-catenin causes focal retention of MD tissue in the epididymides and vasa deferentia. In adult mutant mice, the retained MD tissues express α-smooth muscle actin and desmin, which are markers for uterine differentiation. MD retention inhibited the folding complexity of the developing epididymides and usually led to obstructive azoospermia by spermatoceles. The MDs of urogenital ridges from mutant female embryos showed less regression with added MIS in organ culture compared with control MDs when analyzed by whole mount in situ hybridization for Wnt7a as a marker for the MD epithelium. CA β-catenin did not appear to affect expression of either MIS in the embryonic testes or its type II receptor (AMHR2) in the MD mesenchyme nor did it inhibit pSmad1/5/8 nuclear accumulation, suggesting that dysregulated β-catenin must inhibit MD regression independently of MIS signaling. These studies suggest that dysregulated Wnt/β-catenin signaling in the MD mesenchyme might also be a contributing factor in persistent Müllerian duct syndrome, a form of male pseudohermaphroditism, and development of spermatoceles.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




Similar articles
-
Induction of WNT inhibitory factor 1 expression by Müllerian inhibiting substance/antiMullerian hormone in the Müllerian duct mesenchyme is linked to Müllerian duct regression.Dev Biol. 2014 Feb 1;386(1):227-36. doi: 10.1016/j.ydbio.2013.12.015. Epub 2013 Dec 19. Dev Biol. 2014. PMID: 24362065 Free PMC article.
-
Essential roles of mesenchyme-derived beta-catenin in mouse Müllerian duct morphogenesis.Dev Biol. 2007 Jul 15;307(2):227-36. doi: 10.1016/j.ydbio.2007.04.036. Epub 2007 May 3. Dev Biol. 2007. PMID: 17532316 Free PMC article.
-
β-Catenin is essential for Müllerian duct regression during male sexual differentiation.Development. 2011 May;138(10):1967-75. doi: 10.1242/dev.056143. Epub 2011 Apr 13. Development. 2011. PMID: 21490063 Free PMC article.
-
The in vivo roles of müllerian-inhibiting substance.Curr Top Dev Biol. 1994;29:171-87. doi: 10.1016/s0070-2153(08)60550-5. Curr Top Dev Biol. 1994. PMID: 7828438 Review.
-
Müllerian inhibiting substance: an instructive developmental hormone with diagnostic and possible therapeutic applications.Endocr Rev. 2001 Oct;22(5):657-74. doi: 10.1210/edrv.22.5.0445. Endocr Rev. 2001. PMID: 11588147 Review.
Cited by
-
Induction of WNT inhibitory factor 1 expression by Müllerian inhibiting substance/antiMullerian hormone in the Müllerian duct mesenchyme is linked to Müllerian duct regression.Dev Biol. 2014 Feb 1;386(1):227-36. doi: 10.1016/j.ydbio.2013.12.015. Epub 2013 Dec 19. Dev Biol. 2014. PMID: 24362065 Free PMC article.
-
Partial Müllerian Duct Retention in Smad4 Conditional Mutant Male Mice.Int J Biol Sci. 2016 Apr 21;12(6):667-76. doi: 10.7150/ijbs.12300. eCollection 2016. Int J Biol Sci. 2016. PMID: 27194944 Free PMC article.
-
Development and characterization of human fetal female reproductive tract organoids to understand Müllerian duct anomalies.Proc Natl Acad Sci U S A. 2022 Jul 26;119(30):e2118054119. doi: 10.1073/pnas.2118054119. Epub 2022 Jul 18. Proc Natl Acad Sci U S A. 2022. PMID: 35858415 Free PMC article.
-
Ovarian hormones through Wnt signalling regulate the growth of human and mouse ovarian cancer initiating lesions.Oncotarget. 2016 Oct 4;7(40):64836-64853. doi: 10.18632/oncotarget.11711. Oncotarget. 2016. PMID: 27588493 Free PMC article.
-
Adenomatous polyposis coli (APC) is essential for maintaining the integrity of the seminiferous epithelium.Mol Endocrinol. 2011 Oct;25(10):1725-39. doi: 10.1210/me.2011-0057. Epub 2011 Aug 4. Mol Endocrinol. 2011. PMID: 21816903 Free PMC article.
References
-
- Teixeira J, Maheswaran S, Donahoe PK. Müllerian inhibiting substance: An instructive developmental hormone with diagnostic and possible therapeutic applications. Endocr Rev. 2001;22:657–674. - PubMed
-
- Josso N, Picard JY, Rey R, di Clemente N. Testicular anti-Müllerian hormone: History, genetics, regulation and clinical applications. Pediatr Endocrinol Rev. 2006;3:347–358. - PubMed
-
- Behringer RR, Finegold MJ, Cate RL. Müllerian-inhibiting substance function during mammalian sexual development. Cell. 1994;79:415–425. - PubMed
-
- Jamin SP, Arango NA, Mishina Y, Hanks MC, Behringer RR. Requirement of Bmpr1a for Müllerian duct regression during male sexual development. Nat Genet. 2002;32:408–410. - PubMed
-
- Clarke TR, et al. Mullerian inhibiting substance signaling uses a BMP-like pathway mediated by ALK2 and induces Smad6 expression. Mol Endocrinol. 2001;15:946–959. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

