Dual roles of the sixth transmembrane segment of the CFTR chloride channel in gating and permeation
- PMID: 20805575
- PMCID: PMC2931150
- DOI: 10.1085/jgp.201010480
Dual roles of the sixth transmembrane segment of the CFTR chloride channel in gating and permeation
Abstract
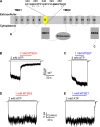
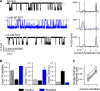
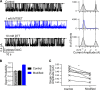
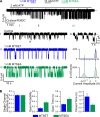
Cystic fibrosis transmembrane conductance regulator (CFTR) is the only member of the adenosine triphosphate-binding cassette (ABC) transporter superfamily that functions as a chloride channel. Previous work has suggested that the external side of the sixth transmembrane segment (TM6) plays an important role in governing chloride permeation, but the function of the internal side remains relatively obscure. Here, on a cysless background, we performed cysteine-scanning mutagenesis and modification to screen the entire TM6 with intracellularly applied thiol-specific methanethiosulfonate reagents. Single-channel amplitude was reduced in seven cysteine-substituted mutants, suggesting a role of these residues in maintaining the pore structure for normal ion permeation. The reactivity pattern of differently charged reagents suggests that the cytoplasmic part of TM6 assumes a secondary structure of an alpha helix, and that reactive sites (341, 344, 345, 348, 352, and 353) reside in two neighboring faces of the helix. Although, as expected, modification by negatively charged reagents inhibits anion permeation, interestingly, modification by positively charged reagents of cysteine thiolates on one face (344, 348, and 352) of the helix affects gating. For I344C and M348C, the open time was prolonged and the closed time was shortened after modification, suggesting that depositions of positive charges at these positions stabilize the open state but destabilize the closed state. For R352C, which exhibited reduced single-channel amplitude, modifications by two positively charged reagents with different chemical properties completely restored the single-channel amplitude but had distinct effects on both the open time and the closed time. These results corroborate the idea that a helix rotation of TM6, which has been proposed to be part of the molecular motions during transport cycles in other ABC transporters, is associated with gating of the CFTR pore.
Figures








References
-
- Alexander C., Ivetac A., Liu X., Norimatsu Y., Serrano J.R., Landstrom A., Sansom M., Dawson D.C. 2009. Cystic fibrosis transmembrane conductance regulator: using differential reactivity toward channel-permeant and channel-impermeant thiol-reactive probes to test a molecular model for the pore. Biochemistry. 48:10078–10088 10.1021/bi901314c - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

