LRCH proteins: a novel family of cytoskeletal regulators
- PMID: 20805893
- PMCID: PMC2923620
- DOI: 10.1371/journal.pone.0012257
LRCH proteins: a novel family of cytoskeletal regulators
Abstract
Background: Comparative genomics has revealed an unexpected level of conservation for gene products across the evolution of animal species. However, the molecular function of only a few proteins has been investigated experimentally, and the role of many animal proteins still remains unknown. Here we report the characterization of a novel family of evolutionary conserved proteins, which display specific features of cytoskeletal scaffolding proteins, referred to as LRCHs.
Principal findings: Taking advantage of the existence of a single LRCH gene in flies, dLRCH, we explored its function in cultured cells, and show that dLRCH act to stabilize the cell cortex during cell division. dLRCH depletion leads to ectopic cortical blebs and alters positioning of the mitotic spindle. We further examined the consequences of dLRCH deletion throughout development and adult life. Although dLRCH is not essential for cell division in vivo, flies lacking dLRCH display a reduced fertility and fitness, particularly when raised at extreme temperatures.
Conclusion/significance: These results support the idea that some cytoskeletal regulators are important to buffer environmental variations and ensure the proper execution of basic cellular processes, such as the control of cell shape, under environmental variations.
Conflict of interest statement
Figures

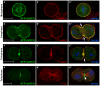
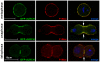
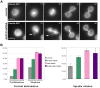


Similar articles
-
Structure and Emerging Functions of LRCH Proteins in Leukocyte Biology.Front Cell Dev Biol. 2020 Sep 22;8:584134. doi: 10.3389/fcell.2020.584134. eCollection 2020. Front Cell Dev Biol. 2020. PMID: 33072765 Free PMC article. Review.
-
Live imaging reveals that the Drosophila actin-binding ERM protein, moesin, co-localizes with the mitotic spindle.Eur J Cell Biol. 2009 Oct;88(10):609-19. doi: 10.1016/j.ejcb.2009.05.006. Epub 2009 Jul 9. Eur J Cell Biol. 2009. PMID: 19592131
-
Studying cytoskeletal dynamics in living cells using green fluorescent protein.Mol Biotechnol. 2002 Jul;21(3):241-50. doi: 10.1385/MB:21:3:241. Mol Biotechnol. 2002. PMID: 12102548 Review.
-
Dynamics of bacterial cytoskeletal elements.Cell Motil Cytoskeleton. 2009 Nov;66(11):909-14. doi: 10.1002/cm.20381. Cell Motil Cytoskeleton. 2009. PMID: 19466751 Review.
-
Control of Lte1 localization by cell polarity determinants and Cdc14.Curr Biol. 2002 Dec 23;12(24):2098-110. doi: 10.1016/s0960-9822(02)01388-x. Curr Biol. 2002. PMID: 12498684
Cited by
-
LRCH1 suppresses migration of CD4+ T cells and refers to disease activity in ulcerative colitis.Int J Med Sci. 2020 Feb 17;17(5):599-608. doi: 10.7150/ijms.39106. eCollection 2020. Int J Med Sci. 2020. PMID: 32210709 Free PMC article.
-
A cell atlas of the adult Drosophila midgut.Proc Natl Acad Sci U S A. 2020 Jan 21;117(3):1514-1523. doi: 10.1073/pnas.1916820117. Epub 2020 Jan 8. Proc Natl Acad Sci U S A. 2020. PMID: 31915294 Free PMC article.
-
LRCH1 interferes with DOCK8-Cdc42-induced T cell migration and ameliorates experimental autoimmune encephalomyelitis.J Exp Med. 2017 Jan;214(1):209-226. doi: 10.1084/jem.20160068. J Exp Med. 2017. PMID: 28028151 Free PMC article.
-
Leucine-rich repeats and calponin homology containing 4 (Lrch4) regulates the innate immune response.J Biol Chem. 2019 Feb 8;294(6):1997-2008. doi: 10.1074/jbc.RA118.004300. Epub 2018 Dec 6. J Biol Chem. 2019. PMID: 30523158 Free PMC article.
-
Knockdown of LRCH4 Remodels Tumor Microenvironment Through Inhibiting YAP and TGF-β/Smad Signaling Pathway in Colorectal Cancer.Comb Chem High Throughput Screen. 2024;27(12):1823-1829. doi: 10.2174/0113862073267943231101065948. Comb Chem High Throughput Screen. 2024. PMID: 38383956
References
-
- Jefford CE, Irminger-Finger I. Mechanisms of chromosome instability in cancers. Crit Rev Oncol Hematol. 2006;59:1–14. - PubMed
-
- Kunda P, Baum B. The actin cytoskeleton in spindle assembly and positioning. Trends Cell Biol. 2009;19:174–179. - PubMed
-
- Glotzer M. The molecular requirements for cytokinesis. Science. 2005;307:1735–1739. - PubMed
-
- Bretscher A, Edwards K, Fehon RG. ERM proteins and merlin: integrators at the cell cortex. Nat Rev Mol Cell Biol. 2002;3:586–599. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

