Gliadin peptide P31-43 localises to endocytic vesicles and interferes with their maturation
- PMID: 20805894
- PMCID: PMC2923621
- DOI: 10.1371/journal.pone.0012246
Gliadin peptide P31-43 localises to endocytic vesicles and interferes with their maturation
Abstract
Background: Celiac Disease (CD) is both a frequent disease (1:100) and an interesting model of a disease induced by food. It consists in an immunogenic reaction to wheat gluten and glutenins that has been found to arise in a specific genetic background; however, this reaction is still only partially understood. Activation of innate immunity by gliadin peptides is an important component of the early events of the disease. In particular the so-called "toxic" A-gliadin peptide P31-43 induces several pleiotropic effects including Epidermal Growth Factor Receptor (EGFR)-dependent actin remodelling and proliferation in cultured cell lines and in enterocytes from CD patients. These effects are mediated by delayed EGFR degradation and prolonged EGFR activation in endocytic vesicles. In the present study we investigated the effects of gliadin peptides on the trafficking and maturation of endocytic vesicles.
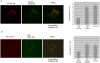
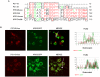
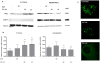
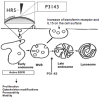
Methods/principal findings: Both P31-43 and the control P57-68 peptide labelled with fluorochromes were found to enter CaCo-2 cells and interact with the endocytic compartment in pulse and chase, time-lapse, experiments. P31-43 was localised to vesicles carrying early endocytic markers at time points when P57-68-carrying vesicles mature into late endosomes. In time-lapse experiments the trafficking of P31-43-labelled vesicles was delayed, regardless of the cargo they were carrying. Furthermore in celiac enterocytes, from cultured duodenal biopsies, P31-43 trafficking is delayed in early endocytic vesicles. A sequence similarity search revealed that P31-43 is strikingly similar to Hrs, a key molecule regulating endocytic maturation. A-gliadin peptide P31-43 interfered with Hrs correct localisation to early endosomes as revealed by western blot and immunofluorescence microscopy.
Conclusions: P31-43 and P57-68 enter cells by endocytosis. Only P31-43 localises at the endocytic membranes and delays vesicle trafficking by interfering with Hrs-mediated maturation to late endosomes in cells and intestinal biopsies. Consequently, in P31-43-treated cells, Receptor Tyrosine Kinase (RTK) activation is extended. This finding may explain the role played by gliadin peptides in inducing proliferation and other effects in enterocytes from CD biopsies.
Conflict of interest statement
Figures





Similar articles
-
Gliadin-mediated proliferation and innate immune activation in celiac disease are due to alterations in vesicular trafficking.PLoS One. 2011 Feb 25;6(2):e17039. doi: 10.1371/journal.pone.0017039. PLoS One. 2011. PMID: 21364874 Free PMC article.
-
An undigested gliadin peptide activates innate immunity and proliferative signaling in enterocytes: the role in celiac disease.Am J Clin Nutr. 2013 Oct;98(4):1123-35. doi: 10.3945/ajcn.112.054544. Epub 2013 Aug 21. Am J Clin Nutr. 2013. PMID: 23966426
-
Growth factor-like activity of gliadin, an alimentary protein: implications for coeliac disease.Gut. 2007 Apr;56(4):480-8. doi: 10.1136/gut.2005.086637. Epub 2006 Aug 4. Gut. 2007. PMID: 16891357 Free PMC article.
-
The gliadin p31-43 peptide: Inducer of multiple proinflammatory effects.Int Rev Cell Mol Biol. 2021;358:165-205. doi: 10.1016/bs.ircmb.2020.10.003. Epub 2020 Nov 5. Int Rev Cell Mol Biol. 2021. PMID: 33707054 Review.
-
Gliadin peptides as triggers of the proliferative and stress/innate immune response of the celiac small intestinal mucosa.Int J Mol Sci. 2014 Nov 7;15(11):20518-37. doi: 10.3390/ijms151120518. Int J Mol Sci. 2014. PMID: 25387079 Free PMC article. Review.
Cited by
-
Latest in vitro and in vivo models of celiac disease.Expert Opin Drug Discov. 2013 Apr;8(4):445-57. doi: 10.1517/17460441.2013.761203. Epub 2013 Jan 8. Expert Opin Drug Discov. 2013. PMID: 23293929 Free PMC article. Review.
-
P31-43, an undigested gliadin peptide, mimics and enhances the innate immune response to viruses and interferes with endocytic trafficking: a role in celiac disease.Sci Rep. 2018 Jul 17;8(1):10821. doi: 10.1038/s41598-018-28830-y. Sci Rep. 2018. PMID: 30018339 Free PMC article.
-
Enterocyte proliferation and signaling are constitutively altered in celiac disease.PLoS One. 2013 Oct 18;8(10):e76006. doi: 10.1371/journal.pone.0076006. eCollection 2013. PLoS One. 2013. PMID: 24204586 Free PMC article.
-
Translational Chemistry Meets Gluten-Related Disorders.ChemistryOpen. 2018 Feb 27;7(3):217-232. doi: 10.1002/open.201700197. eCollection 2018 Mar. ChemistryOpen. 2018. PMID: 29531885 Free PMC article. Review.
-
Endocytosis and transcytosis of gliadin peptides.Mol Cell Pediatr. 2016 Dec;3(1):8. doi: 10.1186/s40348-015-0029-z. Epub 2016 Feb 16. Mol Cell Pediatr. 2016. PMID: 26883352 Free PMC article.
References
-
- Maiuri L, Ciacci C, Ricciardelli I, Vacca L, Raia V, et al. Association between innate response to gliadin and activation of pathogenic T cells in coeliac disease. Lancet. 2003;362:30–7. - PubMed
-
- Anderson RP, Degano P, Godkin AJ, Jewell DP, Hill AV. In vivo antigen challenge in celiac disease identifies a single transglutaminase-modified peptide as the dominant A-gliadin T-cell epitope. Nat Med. 2000;6:337–42. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

