Suppression of uPA and uPAR attenuates angiogenin mediated angiogenesis in endothelial and glioblastoma cell lines
- PMID: 20805979
- PMCID: PMC2929192
- DOI: 10.1371/journal.pone.0012458
Suppression of uPA and uPAR attenuates angiogenin mediated angiogenesis in endothelial and glioblastoma cell lines
Retraction in
-
Retraction: Suppression of uPA and uPAR Attenuates Angiogenin Mediated Angiogenesis in Endothelial and Glioblastoma Cell Lines.PLoS One. 2025 Aug 14;20(8):e0330088. doi: 10.1371/journal.pone.0330088. eCollection 2025. PLoS One. 2025. PMID: 40811436 Free PMC article. No abstract available.
Abstract
Background: In our earlier reports, we showed that downregulation of uPA and uPAR inhibited glioma tumor angiogenesis in SNB19 cells, and intraperitoneal injection of a hairpin shRNA expressing plasmid targeting uPA and uPAR inhibited angiogenesis in nude mice. The exact mechanism by which inhibition of angiogenesis takes place is not clearly understood.
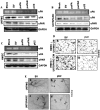
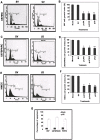
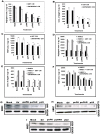
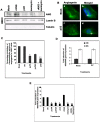
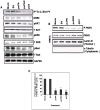
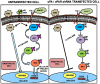
Methodology/principal findings: In the present study, we have attempted to investigate the mechanism by which uPA/uPAR downregulation by shRNA inhibits angiogenesis in endothelial and glioblastoma cell lines. uPA/uPAR downregulation by shRNA in U87 MG and U87 SPARC co-cultures with endothelial cells inhibited angiogenesis as assessed by in vitro angiogenesis assay and in vivo dorsal skin-fold chamber model in nude mice. Protein antibody array analysis of co-cultures of U87 and U87 SPARC cells with endothelial cells treated with pU2 (shRNA against uPA and uPAR) showed decreased angiogenin secretion and angiopoietin-1 as well as several other pro-angiogenic molecules. Therefore, we investigated the role of angiogenin and found that nuclear translocation, ribonucleolytic and 45S rRNA synthesis, which are all critical for angiogenic function of angiogenin, were significantly inhibited in endothelial cells transfected with uPA, uPAR and uPA/uPAR when compared with controls. Moreover, uPA and uPAR downregulation significantly inhibited the phosphorylation of Tie-2 receptor and also down regulated FKHR activation in the nucleus of endothelial cells via the GRB2/AKT/BAD pathway. Treatment of endothelial cells with ruPA increased angiogenin secretion and angiogenin expression as determined by ELISA and western blotting in a dose-dependent manner. The amino terminal fragment of uPA down regulated ruPA-induced angiogenin in endothelial cells, thereby suggesting that uPA plays a critical role in positively regulating angiogenin in glioblastoma cells.
Conclusions/significance: Taken together, our results suggest that uPA/uPAR downregulation suppresses angiogenesis in endothelial cells induced by glioblastoma cell lines partially by downregulation of angiogenin and by inhibition of the angiopoietin-1/AKT/FKHR pathway.
Conflict of interest statement
Figures


Similar articles
-
uPA and uPAR shRNA inhibit angiogenesis via enhanced secretion of SVEGFR1 independent of GM-CSF but dependent on TIMP-1 in endothelial and glioblastoma cells.Mol Oncol. 2012 Feb;6(1):33-47. doi: 10.1016/j.molonc.2011.11.008. Epub 2011 Nov 30. Mol Oncol. 2012. PMID: 22177802 Free PMC article.
-
Specific knockdown of uPA/uPAR attenuates invasion in glioblastoma cells and xenografts by inhibition of cleavage and trafficking of Notch -1 receptor.Mol Cancer. 2011 Oct 17;10:130. doi: 10.1186/1476-4598-10-130. Mol Cancer. 2011. PMID: 22004682 Free PMC article.
-
Suppression of uPAR retards radiation-induced invasion and migration mediated by integrin β1/FAK signaling in medulloblastoma.PLoS One. 2010 Sep 24;5(9):e13006. doi: 10.1371/journal.pone.0013006. PLoS One. 2010. Retraction in: PLoS One. 2025 Jul 15;20(7):e0328091. doi: 10.1371/journal.pone.0328091. PMID: 20886051 Free PMC article. Retracted.
-
The urokinase plasminogen activation system in gastroesophageal cancer: A systematic review and meta-analysis.Oncotarget. 2017 Apr 4;8(14):23099-23109. doi: 10.18632/oncotarget.15485. Oncotarget. 2017. PMID: 28416743 Free PMC article.
-
Systemic pharmacological treatments for chronic plaque psoriasis: a network meta-analysis.Cochrane Database Syst Rev. 2017 Dec 22;12(12):CD011535. doi: 10.1002/14651858.CD011535.pub2. Cochrane Database Syst Rev. 2017. Update in: Cochrane Database Syst Rev. 2020 Jan 9;1:CD011535. doi: 10.1002/14651858.CD011535.pub3. PMID: 29271481 Free PMC article. Updated.
Cited by
-
Adipose-derived mesenchymal stromal cells from aged patients with coronary artery disease keep mesenchymal stromal cell properties but exhibit characteristics of aging and have impaired angiogenic potential.Stem Cells Transl Med. 2014 Jan;3(1):32-41. doi: 10.5966/sctm.2013-0014. Epub 2013 Dec 18. Stem Cells Transl Med. 2014. PMID: 24353175 Free PMC article.
-
Angiogenic Potential and Secretome of Human Apical Papilla Mesenchymal Stem Cells in Various Stress Microenvironments.Stem Cells Dev. 2015 Nov 1;24(21):2496-512. doi: 10.1089/scd.2015.0197. Epub 2015 Sep 2. Stem Cells Dev. 2015. PMID: 26203919 Free PMC article.
-
Reduced Graphene Oxides Modulate the Expression of Cell Receptors and Voltage-Dependent Ion Channel Genes of Glioblastoma Multiforme.Int J Mol Sci. 2021 Jan 6;22(2):515. doi: 10.3390/ijms22020515. Int J Mol Sci. 2021. PMID: 33419226 Free PMC article.
-
Endothelial-specific Enhancer as a Cis Element of PLAUR Regulation by TNF-alpha, IL-1beta, and VEGF.Curr Pharm Des. 2024;30(21):1630-1640. doi: 10.2174/0113816128296376240424072322. Curr Pharm Des. 2024. PMID: 38715331 Review.
-
Suppression of uPA and uPAR blocks radiation-induced MCP-1 mediated recruitment of endothelial cells in meningioma.Cell Signal. 2011 Aug;23(8):1299-310. doi: 10.1016/j.cellsig.2011.03.011. Epub 2011 Mar 21. Cell Signal. 2011. PMID: 21426933 Free PMC article.
References
-
- Yamamoto M, Sawaya R, Mohanam S, Rao VH, Bruner JM, et al. Expression and localization of urokinase-type plasminogen activator receptor in human gliomas. Cancer Res. 1994;54:5016–5020. - PubMed
-
- Murphy G, Atkinson S, Ward R, Gavrilovic J, Reynolds JJ. The role of plasminogen activators in the regulation of connective tissue metalloproteinases. Ann NY Acad Sci. 1992;667:1–12. - PubMed
-
- Rao JS. Molecular mechanisms of glioma invasiveness: the role of proteases. Nat Rev Cancer. 2003;3:489–501. - PubMed
-
- Wei Y, Waltz DA, Rao N, Drummond RJ, Rosenberg S, et al. Identification of the urokinase receptor as an adhesion receptor for vitronectin. J Biol Chem. 1994;269:32380–32388. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

