Evolution of novel signal traits in the absence of female preferences in Neoconocephalus katydids (Orthoptera, Tettigoniidae)
- PMID: 20805980
- PMCID: PMC2929193
- DOI: 10.1371/journal.pone.0012457
Evolution of novel signal traits in the absence of female preferences in Neoconocephalus katydids (Orthoptera, Tettigoniidae)
Abstract
BACKGROUND SIGNIFICANCE: Communication signals that function to bring together the sexes are important for maintaining reproductive isolation in many taxa. Changes in male calls are often attributed to sexual selection, in which female preferences initiate signal divergence. Natural selection can also influence signal traits if calls attract predators or parasitoids, or if calling is energetically costly. Neutral evolution is often neglected in the context of acoustic communication.
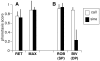
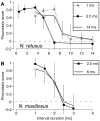
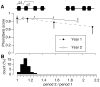
Methodology/principal findings: We describe a signal trait that appears to have evolved in the absence of either sexual or natural selection. In the katydid genus Neoconocephalus, calls with a derived pattern in which pulses are grouped into pairs have evolved five times independently. We have previously shown that in three of these species, females require the double pulse pattern for call recognition, and hence the recognition system of the females is also in a derived state. Here we describe the remaining two species and find that although males produce the derived call pattern, females use the ancestral recognition mechanism in which no pulse pattern is required. Females respond equally well to the single and double pulse calls, indicating that the derived trait is selectively neutral in the context of mate recognition.
Conclusions/significance: These results suggest that 1) neutral changes in signal traits could be important in the diversification of communication systems, and 2) males rather than females may be responsible for initiating signal divergence.
Conflict of interest statement
Figures


References
-
- Greenfield MD. Oxford: Oxford University Press; 2002. Signalers and receivers: Mechanisms and evolution of arthropod communication.432
-
- Gerhardt HC, Huber F. Chicago: University of Chicago Press; 2002. Acoustic Communication in insects and anurans.542
-
- Otte D. Speciation in Hawaiian crickets. In: Otte D, Endler JA, editors. Speciation and its consequences. Sunderland: Sinauer Associates; 1989. pp. 482–526.
-
- Alexander RD, Marshall D C, Cooley JR. Evolutionary perspectives on insect mating. In: Choe JC, Crespi BJ, editors. The evolution of mating systems in insects and arachnids. Cambridge: Cambridge University Press; 1997. pp. 4–31.
-
- Gleason JM, Ritchie MG. Evolution of courtship song and reproductive isolation in the Drosophila willstoni species complex: Do sexual signals diverge the most quickly? Evolution. 1998;52:1493–1500. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Miscellaneous

