13[C]-urea breath test as a novel point-of-care biomarker for tuberculosis treatment and diagnosis
- PMID: 20805989
- PMCID: PMC2929202
- DOI: 10.1371/journal.pone.0012451
13[C]-urea breath test as a novel point-of-care biomarker for tuberculosis treatment and diagnosis
Abstract
Background: Pathogen-specific metabolic pathways may be detected by breath tests based on introduction of stable isotopically-labeled substrates and detection of labeled products in exhaled breath using portable infrared spectrometers.
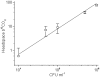
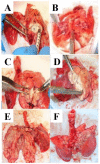
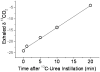
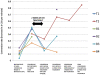
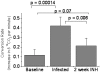
Methodology/principal findings: We tested whether mycobacterial urease activity could be utilized in such a breath test format as the basis of a novel biomarker and diagnostic for pulmonary TB. Sensitized New-Zealand White Rabbits underwent bronchoscopic infection with either Mycobacterium bovis or Mycobacterium tuberculosis. Rabbits were treated with 25 mg/kg of isoniazid (INH) approximately 2 months after infection when significant cavitary lung pathology was present. [(13)C] urea was instilled directly into the lungs of intubated rabbits at selected time points, exhaled air samples analyzed, and the kinetics of delta(13)CO(2) formation were determined. Samples obtained prior to inoculation served as control samples for background (13)CO(2) conversion in the rabbit model. (13)CO(2), from metabolic conversion of [(13)C]-urea by mycobacterial urease activity, was readily detectable in the exhaled breath of infected rabbits within 15 minutes of administration. Analyses showed a rapid increase in the rate of (13)CO(2) formation both early in disease and prior to treatment with INH. Following INH treatment, all evaluable rabbits showed a decrease in the rate of (13)CO(2) formation.
Conclusions/significance: Urea breath testing may provide a useful diagnostic and biomarker assay for tuberculosis and for treatment response. Future work will test specificity for M. tuberculosis using lung-targeted dry powder inhalation formulations, combined with co-administering oral urease inhibitors together with a saturating oral dose of unlabeled urea, which would prevent the delta(13)CO(2) signal from urease-positive gastrointestinal organisms.
Conflict of interest statement
Figures
Similar articles
-
Current tuberculosis diagnostic tools & role of urease breath test.Indian J Med Res. 2012 May;135(5):731-6. Indian J Med Res. 2012. PMID: 22771606 Free PMC article. Review.
-
Rapid in vivo detection of isoniazid-sensitive Mycobacterium tuberculosis by breath test.Nat Commun. 2014 Sep 23;5:4989. doi: 10.1038/ncomms5989. Nat Commun. 2014. PMID: 25247851 Free PMC article.
-
Stool microbiome reveals diverse bacterial ureases as confounders of oral urea breath testing for Helicobacter pylori and Mycobacterium tuberculosis in Bamako, Mali.J Breath Res. 2016 Aug 17;10(3):036012. doi: 10.1088/1752-7155/10/3/036012. J Breath Res. 2016. PMID: 27532494 Free PMC article.
-
In vitro and in vivo studies of a rapid and selective breath test for tuberculosis based upon mycobacterial CO dehydrogenase.mBio. 2014 Apr 15;5(2):e00990. doi: 10.1128/mBio.00990-14. mBio. 2014. PMID: 24736224 Free PMC article.
-
Evaluation of Exalenz Bioscience's BreathID for Helicobacter pylori detection.Expert Rev Mol Diagn. 2015 Mar;15(3):299-312. doi: 10.1586/14737159.2015.982537. Epub 2015 Jan 29. Expert Rev Mol Diagn. 2015. PMID: 25634297 Review.
Cited by
-
Bacterial volatiles and diagnosis of respiratory infections.Adv Appl Microbiol. 2013;82:29-52. doi: 10.1016/B978-0-12-407679-2.00002-8. Adv Appl Microbiol. 2013. PMID: 23415152 Free PMC article. Review.
-
Feasibility and Initial Safety Evaluation of Inhaled 13C-Urea in Ambulatory Patients with Pneumonia.J Aerosol Med Pulm Drug Deliv. 2022 Jan;35(1):57-59. doi: 10.1089/jamp.2021.0050. Epub 2021 Nov 5. J Aerosol Med Pulm Drug Deliv. 2022. PMID: 34748404 Free PMC article. No abstract available.
-
Bacterial urease and its role in long-lasting human diseases.Curr Protein Pept Sci. 2012 Dec;13(8):789-806. doi: 10.2174/138920312804871094. Curr Protein Pept Sci. 2012. PMID: 23305365 Free PMC article. Review.
-
Breath tests in respiratory and critical care medicine: from research to practice in current perspectives.Biomed Res Int. 2013;2013:702896. doi: 10.1155/2013/702896. Epub 2013 Sep 18. Biomed Res Int. 2013. PMID: 24151617 Free PMC article. Review.
-
Detecting virulence and drug-resistance mycobacterial phenotypes in vivo.Trends Microbiol. 2015 Jun;23(6):321-3. doi: 10.1016/j.tim.2015.02.013. Epub 2015 Mar 21. Trends Microbiol. 2015. PMID: 25800730 Free PMC article.
References
-
- Andries K, Verhasselt P, Guillemont J, Göhlmann HW, Neefs JM, et al. A diarylquinoline drug active on the ATP synthase of Mycobacterium tuberculosis. Science. 2005;14:223–227. - PubMed
-
- Stover CK, Warrener P, VanDevanter DR, Sherman DR, Arain TM, et al. A small-molecule nitroimidazopyran drug candidate for the treatment of tuberculosis. Nature. 2000;405:962–966. - PubMed
-
- Perrin FM, Lipman MC, McHugh TD, Gillespie SH. Biomarkers of treatment response in clinical trials of novel antituberculosis agents. Lancet Infect Dis. 2007;7:481–490. - PubMed
-
- Wallis RS, Doherty TM, Onyebujoh P, Vahedi M, Laang, et al. Biomarkers for tuberculosis disease activity, cure, and relapse. Lancet Infect Dis. 2009;9:162–172. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

