Autonomous bioluminescent expression of the bacterial luciferase gene cassette (lux) in a mammalian cell line
- PMID: 20805991
- PMCID: PMC2929204
- DOI: 10.1371/journal.pone.0012441
Autonomous bioluminescent expression of the bacterial luciferase gene cassette (lux) in a mammalian cell line
Abstract
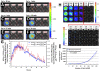
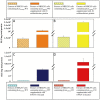
Background: The bacterial luciferase (lux) gene cassette consists of five genes (luxCDABE) whose protein products synergistically generate bioluminescent light signals exclusive of supplementary substrate additions or exogenous manipulations. Historically expressible only in prokaryotes, the lux operon was re-synthesized through a process of multi-bicistronic, codon-optimization to demonstrate for the first time self-directed bioluminescence emission in a mammalian HEK293 cell line in vitro and in vivo.
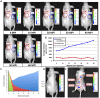
Methodology/principal findings: Autonomous in vitro light production was shown to be 12-fold greater than the observable background associated with untransfected control cells. The availability of reduced riboflavin phosphate (FMNH(2)) was identified as the limiting bioluminescence substrate in the mammalian cell environment even after the addition of a constitutively expressed flavin reductase gene (frp) from Vibrio harveyi. FMNH(2) supplementation led to a 151-fold increase in bioluminescence in cells expressing mammalian codon-optimized luxCDE and frp genes. When injected subcutaneously into nude mice, in vivo optical imaging permitted near instantaneous light detection that persisted independently for the 60 min length of the assay with negligible background.
Conclusions/significance: The speed, longevity, and self-sufficiency of lux expression in the mammalian cellular environment provides a viable and powerful alternative for real-time target visualization not currently offered by existing bioluminescent and fluorescent imaging technologies.
Conflict of interest statement
Figures

References
-
- Contag CH, Bachmann MH. Advances in in vivo bioluminescence imaging of gene expression. Annu Rev Biomed Eng. 2002;4:235–260. - PubMed
-
- Oshiro M. Cooled CCD versus intensified cameras for low-light video - Applications and relative advantages. Methods in Cell Biology. 1998;56:45–62. - PubMed
-
- Tsien RY. The green fluorescent protein. Annu Rev Biochem. 1998;67:509–544. - PubMed
-
- Kalchenko V, Shivtiel S, Malina V, Lapid K, Haramati S, et al. Use of lipophilic near-infrared dye in whole-body optical imaging of hematopoietic cell homing. J Biomed Opt. 2006;11:Article #050507. - PubMed
-
- Bloch S, Lesage F, McIntosh L, Gandjbakhche A, Liang KX, et al. Whole-body fluorescence lifetime imaging of a tumor-targeted near-infrared molecular probe in mice. J Biomed Opt. 2005;10:Article #054003. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

