Insights into neurogenesis and aging: potential therapy for degenerative disease?
- PMID: 20806052
- PMCID: PMC2929019
- DOI: 10.2217/FNL.10.33
Insights into neurogenesis and aging: potential therapy for degenerative disease?
Abstract
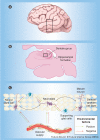
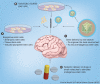
Neurogenesis is the process by which new neural cells are generated from a small population of multipotent stem cells in the adult CNS. This natural generation of new cells is limited in its regenerative capabilities and also declines with age. The use of stem cells in the treatment of neurodegenerative disease may hold great potential; however, the age-related incidence of many CNS diseases coincides with reduced neurogenesis. This review concisely summarizes current knowledge related to adult neurogenesis and its alteration with aging and examines the feasibility of using stem cell and gene therapies to combat diseases of the CNS with advancing age.
Figures
References
-
- Center for Health Workforce Studies . The Impact of the Aging Population on the Health Workforce in the United States: Summary of Key Findings. Center for Health Workforce Studies; NY, USA: 2006. - PubMed
-
- Olanow CW, Stern MB, Sethi K. The scientific and clinical basis for the treatment of Parkinson disease. Neurology. 2009;72(21 Suppl. 4):S1–S136. - PubMed
-
- Kempermann G, Jessberger S, Steiner B, Kronenberg G. Milestones of neuronal development in the adult hippocampus. Trends Neurosci. 2004;27(8):447–452. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
