Human neural stem cells differentiate and promote locomotor recovery in an early chronic spinal cord injury NOD-scid mouse model
- PMID: 20806064
- PMCID: PMC2923623
- DOI: 10.1371/journal.pone.0012272
Human neural stem cells differentiate and promote locomotor recovery in an early chronic spinal cord injury NOD-scid mouse model
Abstract
Background: Traumatic spinal cord injury (SCI) results in partial or complete paralysis and is characterized by a loss of neurons and oligodendrocytes, axonal injury, and demyelination/dysmyelination of spared axons. Approximately 1,250,000 individuals have chronic SCI in the U.S.; therefore treatment in the chronic stages is highly clinically relevant. Human neural stem cells (hCNS-SCns) were prospectively isolated based on fluorescence-activated cell sorting for a CD133(+) and CD24(-/lo) population from fetal brain, grown as neurospheres, and lineage restricted to generate neurons, oligodendrocytes and astrocytes. hCNS-SCns have recently been transplanted sub-acutely following spinal cord injury and found to promote improved locomotor recovery. We tested the ability of hCNS-SCns transplanted 30 days post SCI to survive, differentiate, migrate, and promote improved locomotor recovery.
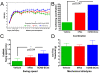
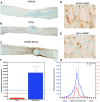
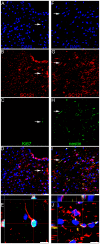
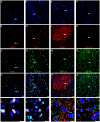
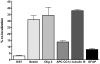
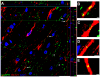
Methods and findings: hCNS-SCns were transplanted into immunodeficient NOD-scid mice 30 days post spinal cord contusion injury. hCNS-SCns transplanted mice demonstrated significantly improved locomotor recovery compared to vehicle controls using open field locomotor testing and CatWalk gait analysis. Transplanted hCNS-SCns exhibited long-term engraftment, migration, limited proliferation, and differentiation predominantly to oligodendrocytes and neurons. Astrocytic differentiation was rare and mice did not exhibit mechanical allodynia. Furthermore, differentiated hCNS-SCns integrated with the host as demonstrated by co-localization of human cytoplasm with discrete staining for the paranodal marker contactin-associated protein.
Conclusions: The results suggest that hCNS-SCns are capable of surviving, differentiating, and promoting improved locomotor recovery when transplanted into an early chronic injury microenvironment. These data suggest that hCNS-SCns transplantation has efficacy in an early chronic SCI setting and thus expands the "window of opportunity" for intervention.
Conflict of interest statement
Figures

Similar articles
-
Immunosuppressants affect human neural stem cells in vitro but not in an in vivo model of spinal cord injury.Stem Cells Transl Med. 2013 Oct;2(10):731-44. doi: 10.5966/sctm.2012-0175. Epub 2013 Aug 27. Stem Cells Transl Med. 2013. PMID: 23981724 Free PMC article.
-
Analysis of host-mediated repair mechanisms after human CNS-stem cell transplantation for spinal cord injury: correlation of engraftment with recovery.PLoS One. 2009 Jun 11;4(6):e5871. doi: 10.1371/journal.pone.0005871. PLoS One. 2009. PMID: 19517014 Free PMC article.
-
Human neural stem cells differentiate and promote locomotor recovery in spinal cord-injured mice.Proc Natl Acad Sci U S A. 2005 Sep 27;102(39):14069-74. doi: 10.1073/pnas.0507063102. Epub 2005 Sep 19. Proc Natl Acad Sci U S A. 2005. PMID: 16172374 Free PMC article.
-
Accelerating locomotor recovery after incomplete spinal injury.Ann N Y Acad Sci. 2013 Mar;1279(1):164-74. doi: 10.1111/nyas.12061. Ann N Y Acad Sci. 2013. PMID: 23531014 Free PMC article. Review.
-
Molecular imaging in stem cell therapy for spinal cord injury.Biomed Res Int. 2014;2014:759514. doi: 10.1155/2014/759514. Epub 2014 Feb 19. Biomed Res Int. 2014. PMID: 24701583 Free PMC article. Review.
Cited by
-
Peptide-modified, hyaluronic acid-based hydrogels as a 3D culture platform for neural stem/progenitor cell engineering.J Biomed Mater Res A. 2019 Apr;107(4):704-718. doi: 10.1002/jbm.a.36603. Epub 2019 Jan 21. J Biomed Mater Res A. 2019. PMID: 30615255 Free PMC article.
-
Transplantation of modified human bone marrow-derived stromal cells affords therapeutic effects on cerebral ischemia in rats.CNS Neurosci Ther. 2022 Dec;28(12):1974-1985. doi: 10.1111/cns.13947. Epub 2022 Aug 24. CNS Neurosci Ther. 2022. PMID: 36000240 Free PMC article.
-
Immunosuppressants affect human neural stem cells in vitro but not in an in vivo model of spinal cord injury.Stem Cells Transl Med. 2013 Oct;2(10):731-44. doi: 10.5966/sctm.2012-0175. Epub 2013 Aug 27. Stem Cells Transl Med. 2013. PMID: 23981724 Free PMC article.
-
Repeated injections of human umbilical cord blood-derived mesenchymal stem cells significantly promotes functional recovery in rabbits with spinal cord injury of two noncontinuous segments.Stem Cell Res Ther. 2018 May 11;9(1):136. doi: 10.1186/s13287-018-0879-0. Stem Cell Res Ther. 2018. PMID: 29751769 Free PMC article.
-
Evaluation of single-dose umbilical cord blood-derived mononuclear cell injection immediately and 7 days after spinal cord trauma in mice.Clinics (Sao Paulo). 2025 Jan 30;80:100579. doi: 10.1016/j.clinsp.2025.100579. eCollection 2025. Clinics (Sao Paulo). 2025. PMID: 39889361 Free PMC article.
References
-
- Okano H, Kaneko S, Okada S, Iwanami A, Nakamura M, et al. Regeneration-based therapies for spinal cord injuries. Neurochem Int. 2007;51:68–73. - PubMed
-
- Mothe AJ, Kulbatski I, Parr A, Mohareb M, Tator CH. Adult spinal cord stem/progenitor cells transplanted as neurospheres preferentially differentiate into oligodendrocytes in the adult rat spinal cord. Cell Transplant. 2008;17:735–751. - PubMed
-
- Houle JD, Tessler A. Repair of chronic spinal cord injury. Exp Neurol. 2003;182:247–260. - PubMed
-
- Cummings BJ, Hooshmand M, Salazar DL, Anderson AJ. Ribak CE, editor. Human neural stem cell-mediated repair of the contused spinal cord: Timing the microenvironment. From Development to Degeneration and Regeneration of the Nervous System: Oxford University Press. 2008. pp. 297–322.
-
- Fawcett JW, Curt A, Steeves JD, Coleman WP, Tuszynski MH, et al. Guidelines for the conduct of clinical trials for spinal cord injury as developed by the ICCP panel: spontaneous recovery after spinal cord injury and statistical power needed for therapeutic clinical trials. Spinal Cord. 2007;45:190–205. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

