Characterization of 1H NMR signal in human cortical bone for magnetic resonance imaging
- PMID: 20806375
- PMCID: PMC2933073
- DOI: 10.1002/mrm.22459
Characterization of 1H NMR signal in human cortical bone for magnetic resonance imaging
Abstract
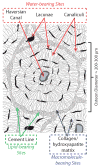
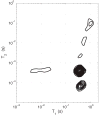
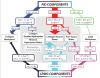
Recent advancements in MRI have enabled clinical imaging of human cortical bone, providing a potentially powerful new means for assessing bone health with molecular-scale sensitivities unavailable to conventional X-ray-based diagnostics. In human cortical bone, MRI is sensitive to populations of protons ((1)H) partitioned among water and protein sources, which may be differentiated according to intrinsic NMR properties such as chemical shift and transverse and longitudinal relaxation rates. Herein, these NMR properties were assessed in human cortical bone donors from a broad age range, and four distinct (1)H populations were consistently identified and attributed to five microanatomical sources. These findings show that modern human cortical bone MRI contrast will be dominated by collagen-bound water, which can also be exploited to study human cortical bone collagen via magnetization transfer.
2010 Wiley-Liss, Inc.
Figures
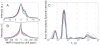


References
-
- Johnell O, Kanis JA, Oden A, Johansson H, De Laet C, Delmas P, Eisman JA, Fujiwara S, Kroger H, Mellstrom D, Meunier PJ, Melton LJ, O’Neill T, Pols H, Reeve J, Silman A, Tenenhouse A. Predictive value of BMD for hip and other fractures. J Bone Miner Res. 2005;20(7):1185–1194. - PubMed
-
- Bembey AK, Bushby AJ, Boyde A, Ferguson VL, Oyen ML. Hydration effects on the micro-mechanical properties of bone. J Mater Res. 2006;21(8):1962–1968.
-
- Yan JH, Daga A, Kumar R, Mecholsky JJ. Fracture toughness and work of fracture of hydrated, dehydrated, and ashed bovine bone. J Biomech. 2008;41(9):1929–1936. - PubMed
-
- Sasaki N, Enyo A. Viscoelastic properties of bone as a function of water-content. J Biomech. 1995;28(7):809–815. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

