Normal canine brain maturation at magnetic resonance imaging
- PMID: 20806866
- PMCID: PMC2936715
- DOI: 10.1111/j.1740-8261.2010.01681.x
Normal canine brain maturation at magnetic resonance imaging
Abstract
The normal neonatal canine brain exhibits marked differences from that of the mature brain. With development into adulthood, there is a decrease in relative water content and progressive myelination; these changes are observable with magnetic resonance imaging (MRI) and are characterized by a repeatable and predictable time course. We characterized these developmental changes on common MRI sequences and identified clinically useful milestones of transition. To accomplish this, 17 normal dogs underwent MRI of the brain at various times after birth from 1 to 36 weeks. Sequences acquired were T1-weighted (T1W), T2-weighted (T2W), fluid attenuated inversion recovery, short tau inversion recovery, and diffusion weighted imaging sequences. The images were assessed subjectively for gray and white matter relative signal intensity and results correlated with histologic findings. The development of the neonatal canine brain follows a pattern that qualitatively matches that observed in humans, and which can be characterized adequately on T1W and T2W images. At birth, the relative gray matter to white matter signal intensity of the cortex is reversed from that of the adult with an isointense transition at 3-4 weeks on T1W and 4-8 weeks on T2W images. This is followed by the expected mature gray matter to white matter relative intensity that undergoes continued development to a mostly adult appearance by 16 weeks. On the fluid attenuated inversion recovery sequence, the cortical gray and white matter exhibit an additional signal intensity reversal during the juvenile period that is due to the initial high relative water content at the subcortical white matter, with its marked T1 relaxation effect.
Figures
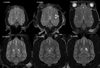

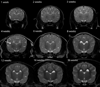

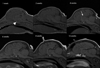

















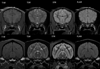
References
-
- Fox MW. Integrative development of brain and behavior in the dog. Chicago and London: The Univerisity of Chicago Press; 1971.
-
- Baratti C, Barnett AS, Pierpaoli C. Comparitive MR imaging study of brain maturation in kittens with T1, T2, and the trace of the diffusion tensor. Radiology. 1999;210:133–142. - PubMed
-
- Barkovich AJ, Kjos BO, Jackson DE, Jr, Norman D. Normal maturation of the neonatal and infant brain: MR imaging at 1.5 T. Radiology. 1988;166:173–180. - PubMed
-
- Miot-Noirault E, Barantin L, Akoka S, Le Pape A. T2 relaxation time as a marker of brain myelination: experimental MR study in two neonatal animal models. J Neurosci Methods. 1997;72:5–14. - PubMed
-
- Jacobson S. Sequence of Myelinization in the Brain of the Albino Rat. A. Cerebral Cortex, Thalamus and Related Structures. J Comp Neurol. 1963;121:5–29. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

