Highly enantioselective catalytic dynamic resolution of N-Boc-2-lithiopiperidine: synthesis of (R)-(+)-N-Boc-pipecolic acid, (S)-(-)-coniine, (S)-(+)-pelletierine, (+)-beta-conhydrine, and (S)-(-)-ropivacaine and formal synthesis of (-)-lasubine II and (+)-cermizine C
- PMID: 20806976
- PMCID: PMC2945905
- DOI: 10.1021/ja105772z
Highly enantioselective catalytic dynamic resolution of N-Boc-2-lithiopiperidine: synthesis of (R)-(+)-N-Boc-pipecolic acid, (S)-(-)-coniine, (S)-(+)-pelletierine, (+)-beta-conhydrine, and (S)-(-)-ropivacaine and formal synthesis of (-)-lasubine II and (+)-cermizine C
Abstract
The catalytic dynamic resolution (CDR) of rac-2-lithio-N-Boc-piperidine using chiral ligand 8 or its diastereomer 9 in the presence of TMEDA has led to the highly enantioselective syntheses of both enantiomers of 2-substituted piperidines using a wide range of electrophiles. The CDR has been applied to the synthesis of (R)- and (S)-pipecolic acid derivatives, (+)-beta-conhydrine, (S)-(+)-pelletierine, and (S)-(-)-ropivacaine and the formal synthesis of (-)-lasubine II and (+)-cermizine C.
Figures

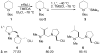
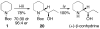

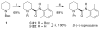
Similar articles
-
Preparation of enantiopure substituted piperidines containing 2-alkene or 2-alkyne chains: application to total syntheses of natural quinolizidine-alkaloids.J Org Chem. 2010 Mar 19;75(6):1911-6. doi: 10.1021/jo902615u. J Org Chem. 2010. PMID: 20155953
-
Application of catalytic dynamic resolution of N-Boc-2-lithiopiperidine to the asymmetric synthesis of 2-aryl and 2-vinyl piperidines.Org Lett. 2011 Feb 4;13(3):394-7. doi: 10.1021/ol102682r. Epub 2010 Dec 21. Org Lett. 2011. PMID: 21174392 Free PMC article.
-
Asymmetric substitutions of 2-lithiated N-boc-piperidine and N-Boc-azepine by dynamic resolution.Chemistry. 2010 Apr 6;16(13):4082-90. doi: 10.1002/chem.200903059. Chemistry. 2010. PMID: 20175161
-
Selective methodologies for the synthesis of biologically active piperidinic compounds.Chem Rec. 2005;5(2):70-80. doi: 10.1002/tcr.20035. Chem Rec. 2005. PMID: 15825169 Review.
-
[Asymmetric synthesis using chiral bases].Yakugaku Zasshi. 1997 Nov;117(10-11):800-16. doi: 10.1248/yakushi1947.117.10-11_800. Yakugaku Zasshi. 1997. PMID: 9414592 Review. Japanese.
Cited by
-
Dynamics of catalytic resolution of 2-lithio-N-Boc-piperidine by ligand exchange.J Am Chem Soc. 2012 Oct 10;134(40):16845-55. doi: 10.1021/ja307796e. Epub 2012 Sep 25. J Am Chem Soc. 2012. PMID: 22967289 Free PMC article.
-
Enantioselective approach to quinolizidines: total synthesis of cermizine D and formal syntheses of senepodine G and cermizine C.J Org Chem. 2013 May 17;78(10):4779-800. doi: 10.1021/jo400324t. Epub 2013 Apr 29. J Org Chem. 2013. PMID: 23627426 Free PMC article.
-
Direct Catalytic N-Alkylation of α-Amino Acid Esters and Amides Using Alcohols with High Retention of Stereochemistry.ChemSusChem. 2021 Jun 8;14(11):2303-2307. doi: 10.1002/cssc.202100373. Epub 2021 May 7. ChemSusChem. 2021. PMID: 33961350 Free PMC article.
-
Electrochemical Vicinal C-H Difunctionalization of Saturated Azaheterocycles.J Am Chem Soc. 2024 Jan 24;146(3):1794-1798. doi: 10.1021/jacs.3c12336. Epub 2024 Jan 8. J Am Chem Soc. 2024. PMID: 38190508 Free PMC article.
-
Catalytic asymmetric carbon-carbon bond formation via allylic alkylations with organolithium compounds.Nat Chem. 2011 May;3(5):377-81. doi: 10.1038/nchem.1009. Epub 2011 Mar 13. Nat Chem. 2011. PMID: 21505496
References
-
- Beak P, Lee WK. Tetrahedron Lett. 1989;30:1197.
-
- Kerrick ST, Beak P. J Am Chem Soc. 1991;113:9708.
-
- Bailey WF, Beak P, Kerrick ST, Ma S, Wiberg KB. J Am Chem Soc. 2002;124:1889. - PubMed
-
- Stead D, Carbone G, O’Brien P, Campos KR, Coldham I, Sanderson A. J Am Chem Soc. 2010;132:7260. - PubMed
-
- Coldham I, Raimbault S, Chovatia PT, Patel JJ, Leonori D, Sheikh NS, Whittaker DTE. Chem Commun. 2008:4174. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

