The MYST family histone acetyltransferase regulates gene expression and cell cycle in malaria parasite Plasmodium falciparum
- PMID: 20807207
- PMCID: PMC2978264
- DOI: 10.1111/j.1365-2958.2010.07371.x
The MYST family histone acetyltransferase regulates gene expression and cell cycle in malaria parasite Plasmodium falciparum
Abstract
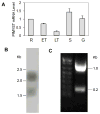
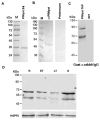
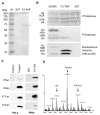
Histone lysine acetylation, normally associated with euchromatin and active genes, is regulated by different families of histone acetyltransferases (HATs). A single Plasmodium falciparum MYST (PfMYST) HAT was expressed as a long and a short version in intraerythrocytic stages. Whereas the recombinant PfMYST expressed in prokaryotes and insect cells did not show HAT activity, recombinant PfMYST purified from the parasites exhibited a predilection to acetylate histone H4 in vitro at K5, K8, K12 and K16. Tagging PfMYST with the green fluorescent protein at the C-terminus showed that PfMYST protein was localized in both the nucleus and cytoplasm. Consistent with the importance of H4 acetylation in var gene expression, PfMYST was recruited to the active var promoter. Attempts to disrupt PfMYST were not successful, suggesting that PfMYST is essential for asexual intraerythrocytic growth. However, overexpression of the long, active or a truncated, non-active version of PfMYST by stable integration of the expression cassette in the parasite genome resulted in changes of H4 acetylation and cell cycle progression. Furthermore, parasites with PfMYST overexpression showed changes in sensitivity to DNA-damaging agents. Collectively, this study showed that PfMYST plays important roles in cellular processes such as gene activation, cell cycle control and DNA repair.
© 2010 Blackwell Publishing Ltd.
Figures








Similar articles
-
MYST regulates DNA repair and forms a NuA4-like complex in the malaria parasite Plasmodium falciparum.mSphere. 2024 Apr 23;9(4):e0014024. doi: 10.1128/msphere.00140-24. Epub 2024 Apr 2. mSphere. 2024. PMID: 38564734 Free PMC article.
-
Role of PfMYST in DNA replication in Plasmodium falciparum.Exp Parasitol. 2022 Nov;242:108396. doi: 10.1016/j.exppara.2022.108396. Epub 2022 Oct 11. Exp Parasitol. 2022. PMID: 36228701
-
Inhibition of PfMYST Histone Acetyltransferase Activity Blocks Plasmodium falciparum Growth and Survival.Antimicrob Agents Chemother. 2020 Dec 16;65(1):e00953-20. doi: 10.1128/AAC.00953-20. Print 2020 Dec 16. Antimicrob Agents Chemother. 2020. PMID: 33046499 Free PMC article.
-
Plasmodium falciparum: epigenetic control of var gene regulation and disease.Subcell Biochem. 2013;61:659-82. doi: 10.1007/978-94-007-4525-4_28. Subcell Biochem. 2013. PMID: 23150271 Review.
-
MYST-family histone acetyltransferases: beyond chromatin.Cell Mol Life Sci. 2011 Apr;68(7):1147-56. doi: 10.1007/s00018-010-0599-9. Epub 2010 Dec 4. Cell Mol Life Sci. 2011. PMID: 21132344 Free PMC article. Review.
Cited by
-
PfSWIB, a potential chromatin regulator for var gene regulation and parasite development in Plasmodium falciparum.Parasit Vectors. 2020 Feb 4;13(1):48. doi: 10.1186/s13071-020-3918-5. Parasit Vectors. 2020. PMID: 32019597 Free PMC article.
-
DNA damage regulation and its role in drug-related phenotypes in the malaria parasites.Sci Rep. 2016 Apr 1;6:23603. doi: 10.1038/srep23603. Sci Rep. 2016. PMID: 27033103 Free PMC article.
-
HAT3-mediated acetylation of PCNA precedes PCNA monoubiquitination following exposure to UV radiation in Leishmania donovani.Nucleic Acids Res. 2015 Jun 23;43(11):5423-41. doi: 10.1093/nar/gkv431. Epub 2015 May 6. Nucleic Acids Res. 2015. PMID: 25948582 Free PMC article.
-
Characterization of the dual role of Plasmodium falciparum DNA methyltransferase in regulating transcription and translation.Nucleic Acids Res. 2023 May 8;51(8):3918-3933. doi: 10.1093/nar/gkad248. Nucleic Acids Res. 2023. PMID: 37026483 Free PMC article.
-
Population genomics and transcriptomics of Plasmodium falciparum in Cambodia and Vietnam uncover key components of the artemisinin resistance genetic background.Nat Commun. 2024 Dec 5;15(1):10625. doi: 10.1038/s41467-024-54915-6. Nat Commun. 2024. PMID: 39639029 Free PMC article.
References
-
- Adachi N, Kimura A, Horikoshi M. A conserved motif common to the histone acetyltransferase Esa1 and the histone deacetylase Rpd3. J Biol Chem. 2002;277:35688–35695. - PubMed
-
- Akhtar A, Zink D, Becker PB. Chromodomains are protein-RNA interaction modules. Nature. 2000;407:405–409. - PubMed
-
- Alkhalil A, Achur RN, Valiyaveettil M, Ockenhouse CF, Gowda DC. Structural requirements for the adherence of Plasmodium falciparum-infected erythrocytes to chondroitin sulfate proteoglycans of human placenta. J Biol Chem. 2000;275:40357–40364. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

