Hsp72 mediates TAp73α anti-apoptotic effects in small cell lung carcinoma cells
- PMID: 20807285
- PMCID: PMC4373366
- DOI: 10.1111/j.1582-4934.2010.01166.x
Hsp72 mediates TAp73α anti-apoptotic effects in small cell lung carcinoma cells
Erratum in
-
Corrigendum.J Cell Mol Med. 2017 Feb;21(2):418. doi: 10.1111/jcmm.13094. J Cell Mol Med. 2017. PMID: 28121075 Free PMC article. No abstract available.
Abstract
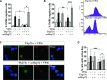
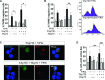
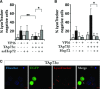
The transcription factor p73, a member of the p53 family of proteins, is involved in the regulation of cell cycle progression and apoptosis. Due to alternative promoters and carboxy-terminal splicing, the P73 gene gives rise to a range of different isoforms. Interestingly, a particular increase in expression of the TAp73α isoform has been reported in various tumours. In addition, TAp73α has been shown to inhibit Bax activation and mitochondrial dysfunctions and thereby to confer small cell lung carcinoma (SCLC) cells resistance to drug-induced apoptosis. However, the precise mechanism by which TAp73α exerts its pro-survival effect is yet unclear. Here we report that TAp73α, but not TAp73β, regulates the expression of inducible Hsp72/HSPA1A. Hsp72 proved to be required for the survival effects of TAp73α as antisense knockdown of Hsp72 resulted in an abolishment of the anti-apoptotic effect of TAp73α in SCLC cells upon Etoposide treatment. Importantly, depletion of Hsp72 allowed activation of Bax, loss of mitochondrial membrane potential and lysosomal membrane permeabilization in SCLC cells even in the presence of TAp73α. Finally, we revealed that TAp73β counteracts the anti-apoptotic effect of TAp73α by preventing Hsp72 induction. Our results thus provide additional evidence for the potential oncogenic role of TAp73α, and extend the understanding of the mechanism for its anti-apoptotic effect.
© 2011 The Authors Journal compilation © 2011 Foundation for Cellular and Molecular Medicine/Blackwell Publishing Ltd.
Figures




Similar articles
-
TAp73alpha protects small cell lung carcinoma cells from caspase-2 induced mitochondrial mediated apoptotic cell death.Oncotarget. 2011 Dec;2(12):1145-54. doi: 10.18632/oncotarget.391. Oncotarget. 2011. PMID: 22201672 Free PMC article.
-
TAp73β-mediated suppression of cell migration requires p57Kip2 control of actin cytoskeleton dynamics.Oncotarget. 2013 Feb;4(2):289-97. doi: 10.18632/oncotarget.833. Oncotarget. 2013. PMID: 23470527 Free PMC article.
-
Crosstalk between c-Jun and TAp73alpha/beta contributes to the apoptosis-survival balance.Nucleic Acids Res. 2011 Aug;39(14):6069-85. doi: 10.1093/nar/gkr028. Epub 2011 Mar 31. Nucleic Acids Res. 2011. PMID: 21459846 Free PMC article.
-
Role of p73 in malignancy: tumor suppressor or oncogene?Cell Death Differ. 2002 Mar;9(3):237-45. doi: 10.1038/sj.cdd.4400995. Cell Death Differ. 2002. PMID: 11859406 Review.
-
p53/p63/p73 isoforms: an orchestra of isoforms to harmonise cell differentiation and response to stress.Cell Death Differ. 2006 Jun;13(6):962-72. doi: 10.1038/sj.cdd.4401914. Cell Death Differ. 2006. PMID: 16601753 Review.
Cited by
-
TAp73alpha protects small cell lung carcinoma cells from caspase-2 induced mitochondrial mediated apoptotic cell death.Oncotarget. 2011 Dec;2(12):1145-54. doi: 10.18632/oncotarget.391. Oncotarget. 2011. PMID: 22201672 Free PMC article.
-
DLC2 inhibits development of glioma through regulating the expression ratio of TAp73α/TAp73β.Am J Cancer Res. 2018 Jul 1;8(7):1200-1213. eCollection 2018. Am J Cancer Res. 2018. PMID: 30094094 Free PMC article.
-
Identification of prognostic and bone metastasis-related alternative splicing signatures in mesothelioma.Cancer Med. 2021 Jul;10(13):4478-4492. doi: 10.1002/cam4.3977. Epub 2021 May 26. Cancer Med. 2021. PMID: 34041868 Free PMC article.
-
Corrigendum.J Cell Mol Med. 2017 Feb;21(2):418. doi: 10.1111/jcmm.13094. J Cell Mol Med. 2017. PMID: 28121075 Free PMC article. No abstract available.
-
TAp73β-mediated suppression of cell migration requires p57Kip2 control of actin cytoskeleton dynamics.Oncotarget. 2013 Feb;4(2):289-97. doi: 10.18632/oncotarget.833. Oncotarget. 2013. PMID: 23470527 Free PMC article.
References
-
- Kaghad M, Bonnet H, Yang A, et al. Monoallelically expressed gene related to p53 at 1p36, a region frequently deleted in neuroblastoma and other human cancers. Cell. 1997;90:809–19. - PubMed
-
- Jost CA, Marin MC, Kaelin WG., Jr p73 is a simian [correction of human] p53-related protein that can induce apoptosis. Nature. 1997;389:191–4. - PubMed
-
- De Laurenzi V, Raschella G, Barcaroli D, et al. Induction of neuronal differentiation by p73 in a neuroblastoma cell line. J Biol Chem. 2000;275:15226–31. - PubMed
-
- Grob TJ, Novak U, Maisse C, et al. Human delta Np73 regulates a dominant negative feedback loop for TAp73 and p53. Cell Death Differ. 2001;8:1213–23. - PubMed
-
- Chi SG, Chang SG, Lee SJ, et al. Elevated and biallelic expression of p73 is associated withprogression of human bladder cancer. Cancer Res. 1999;59:2791–3. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

