Oestrogen blocks the nuclear entry of SOX9 in the developing gonad of a marsupial mammal
- PMID: 20807406
- PMCID: PMC2940779
- DOI: 10.1186/1741-7007-8-113
Oestrogen blocks the nuclear entry of SOX9 in the developing gonad of a marsupial mammal
Abstract
Background: Hormones are critical for early gonadal development in nonmammalian vertebrates, and oestrogen is required for normal ovarian development. In contrast, mammals determine sex by the presence or absence of the SRY gene, and hormones are not thought to play a role in early gonadal development. Despite an XY sex-determining system in marsupial mammals, exposure to oestrogen can override SRY and induce ovarian development of XY gonads if administered early enough. Here we assess the effect of exogenous oestrogen on the molecular pathways of mammalian gonadal development.
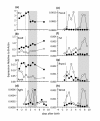
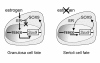
Results: We examined the expression of key testicular (SRY, SOX9, AMH and FGF9) and ovarian (WNT4, RSPO1, FOXL2 and FST) markers during gonadal development in the marsupial tammar wallaby (Macropus eugenii) and used these data to determine the effect of oestrogen exposure on gonadal fate. During normal development, we observed male specific upregulation of AMH and SOX9 as in the mouse and human testis, but this upregulation was initiated before the peak in SRY expression and 4 days before testicular cord formation. Similarly, key genes for ovarian development in mouse and human were also upregulated during ovarian differentiation in the tammar. In particular, there was early sexually dimorphic expression of FOXL2 and WNT4, suggesting that these genes are key regulators of ovarian development in all therian mammals. We next examined the effect of exogenous oestrogen on the development of the mammalian XY gonad. Despite the presence of SRY, exogenous oestrogen blocked the key male transcription factor SOX9 from entering the nuclei of male somatic cells, preventing activation of the testicular pathway and permitting upregulation of key female genes, resulting in ovarian development of the XY gonad.
Conclusions: We have uncovered a mechanism by which oestrogen can regulate gonadal development through the nucleocytoplasmic shuttling of SOX9. This may represent an underlying ancestral mechanism by which oestrogen promotes ovarian development in the gonads of nonmammalian vertebrates. Furthermore, oestrogen may retain this function in adult female mammals to maintain granulosa cell fate in the differentiated ovary by suppressing nuclear translocation of the SOX9 protein. See commentary: http://www.biomedcentral.com/1741-7007/8/110.
Figures



Comment in
-
Oestrogen shuts the door on SOX9.BMC Biol. 2010 Aug 31;8:110. doi: 10.1186/1741-7007-8-110. BMC Biol. 2010. PMID: 20828373 Free PMC article.
Similar articles
-
A role for estrogen in somatic cell fate of the mammalian gonad.Chromosome Res. 2012 Jan;20(1):239-45. doi: 10.1007/s10577-011-9260-1. Chromosome Res. 2012. PMID: 22161125 Review.
-
Mouse Gonad Development in the Absence of the Pro-Ovary Factor WNT4 and the Pro-Testis Factor SOX9.Cells. 2020 Apr 29;9(5):1103. doi: 10.3390/cells9051103. Cells. 2020. PMID: 32365547 Free PMC article.
-
Inefficient Sox9 upregulation and absence of Rspo1 repression lead to sex reversal in the B6.XYTIR mouse gonad†.Biol Reprod. 2024 May 9;110(5):985-999. doi: 10.1093/biolre/ioae018. Biol Reprod. 2024. PMID: 38376238 Free PMC article.
-
Prostaglandin D2 Regulates SOX9 Nuclear Translocation during Gonadal Sex Determination in Tammar Wallaby, Macropus eugenii.Sex Dev. 2017;11(3):143-150. doi: 10.1159/000473782. Epub 2017 May 5. Sex Dev. 2017. PMID: 28472794
-
Exogenous Oestrogen Impacts Cell Fate Decision in the Developing Gonads: A Potential Cause of Declining Human Reproductive Health.Int J Mol Sci. 2020 Nov 8;21(21):8377. doi: 10.3390/ijms21218377. Int J Mol Sci. 2020. PMID: 33171657 Free PMC article. Review.
Cited by
-
Loss of WNT4 in the gubernaculum causes unilateral cryptorchidism and fertility defects.Development. 2022 Dec 1;149(23):dev201093. doi: 10.1242/dev.201093. Epub 2022 Nov 30. Development. 2022. PMID: 36448532 Free PMC article.
-
A role for estrogen in somatic cell fate of the mammalian gonad.Chromosome Res. 2012 Jan;20(1):239-45. doi: 10.1007/s10577-011-9260-1. Chromosome Res. 2012. PMID: 22161125 Review.
-
Oestrogen Activates the MAP3K1 Cascade and β-Catenin to Promote Granulosa-like Cell Fate in a Human Testis-Derived Cell Line.Int J Mol Sci. 2021 Sep 17;22(18):10046. doi: 10.3390/ijms221810046. Int J Mol Sci. 2021. PMID: 34576208 Free PMC article.
-
Avian Sex Determination: A Chicken and Egg Conundrum.Sex Dev. 2023;17(2-3):120-133. doi: 10.1159/000529754. Epub 2023 Feb 16. Sex Dev. 2023. PMID: 36796340 Free PMC article. Review.
-
Oestrogen shuts the door on SOX9.BMC Biol. 2010 Aug 31;8:110. doi: 10.1186/1741-7007-8-110. BMC Biol. 2010. PMID: 20828373 Free PMC article.
References
-
- Bagheri-Fam S, Sinclair AH, Koopman P, Harley VR. Conserved regulatory modules in the Sox9 testis-specific enhancer predict roles for SOX TCF/LEF Forkhead DMRT and GATA proteins in vertebrate sex determination. Int J Biochem Cell Biol. 2009;42:472–477. doi: 10.1016/j.biocel.2009.07.001. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

