How common is ecological speciation in plant-feeding insects? A 'Higher' Nematinae perspective
- PMID: 20807452
- PMCID: PMC2936908
- DOI: 10.1186/1471-2148-10-266
How common is ecological speciation in plant-feeding insects? A 'Higher' Nematinae perspective
Abstract
Background: Ecological speciation is a process in which a transiently resource-polymorphic species divides into two specialized sister lineages as a result of divergent selection pressures caused by the use of multiple niches or environments. Ecology-based speciation has been studied intensively in plant-feeding insects, in which both sympatric and allopatric shifts onto novel host plants could speed up diversification. However, while numerous examples of species pairs likely to have originated by resource shifts have been found, the overall importance of ecological speciation in relation to other, non-ecological speciation modes remains unknown. Here, we apply phylogenetic information on sawflies belonging to the 'Higher' Nematinae (Hymenoptera: Tenthredinidae) to infer the frequency of niche shifts in relation to speciation events.
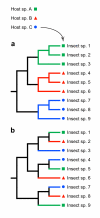
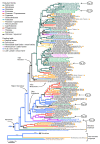
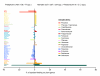
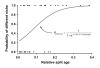
Results: Phylogenetic trees reconstructed on the basis of DNA sequence data show that the diversification of higher nematines has involved frequent shifts in larval feeding habits and in the use of plant taxa. However, the inferred number of resource shifts is considerably lower than the number of past speciation events, indicating that the majority of divergences have occurred by non-ecological allopatric speciation; based on a time-corrected analysis of sister species, we estimate that a maximum of c. 20% of lineage splits have been triggered by a change in resource use. In addition, we find that postspeciational changes in geographic distributions have led to broad sympatry in many species having identical host-plant ranges.
Conclusion: Our analysis indicates that the importance of niche shifts for the diversification of herbivorous insects is at present implicitly and explicitly overestimated. In the case of the Higher Nematinae, employing a time correction for sister-species comparisons lowered the proportion of apparent ecology-based speciation events from c. 50-60% to around 20%, but such corrections are still lacking in other herbivore groups. The observed convergent but asynchronous shifting among dominant northern plant taxa in many higher-nematine clades, in combination with the broad overlaps in the geographic distributions of numerous nematine species occupying near-identical niches, indicates that host-plant shifts and herbivore community assembly are largely unconstrained by direct or indirect competition among species. More phylogeny-based studies on connections between niche diversification and speciation are needed across many insect taxa, especially in groups that exhibit few host shifts in relation to speciation.
Figures


References
-
- Schluter D. The Ecology of Adaptive Radiation. Oxford, Oxford University Press; 2000.
-
- Rundle HD, Nosil P. Ecological speciation. Ecol Lett. 2005;8:336–352. doi: 10.1111/j.1461-0248.2004.00715.x. - DOI
-
- Smith TB, Skúlason S. Evolutionary significance of resource polymorphisms in fishes, amphibians, and birds. Annu Rev Ecol Syst. 1996;27:111–133. doi: 10.1146/annurev.ecolsys.27.1.111. - DOI
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Miscellaneous

