An okadaic acid-induced model of tauopathy and cognitive deficiency
- PMID: 20807517
- PMCID: PMC6980113
- DOI: 10.1016/j.brainres.2010.08.077
An okadaic acid-induced model of tauopathy and cognitive deficiency
Abstract
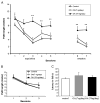
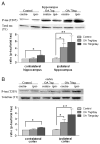
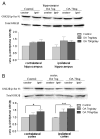
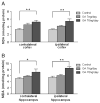
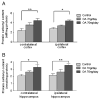
Alzheimer's disease (AD) is a progressive neurodegenerative disease that causes cognitive and behavioral deterioration in the elderly. Neurofibrillary tangles (NFTs) are one of the pathological hallmarks of AD that has been shown to correlate positively with the severity of dementia in the neocortex of AD patients. In an attempt to characterize an in vivo AD tauopathy model, okadaic acid (OA), a protein phosphatase inhibitor, was microinfused into the right lateral dorsal hippocampus area of ovariectomized adult rat. Cognitive deficiency was seen in OA-treated rats without a change in motor function. Both silver staining and immunohistochemistry staining revealed that OA treatment induces NFTs-like conformational changes in both the cortex and hippocampus. Phosphorylated tau as well as cyclin-dependent kinase 5 (cdk5) and its coactivator, p25, were significantly increased in these regions of the brain. Oxidative stress was also increased with OA treatment as measured by protein carbonylation and lipid peroxidation. These data suggest that the unilateral microinfusion of OA into the dorsal hippocampus causes cognitive deficiency, NFTs-like pathological changes, and oxidative stress as seen in AD pathology via tau hyperphosphorylation caused by inhibition of protein phosphatases.
Copyright © 2010. Published by Elsevier B.V.
Figures





Similar articles
-
Neuroprotective Studies of Evodiamine in an Okadaic Acid-Induced Neurotoxicity.Int J Mol Sci. 2021 May 19;22(10):5347. doi: 10.3390/ijms22105347. Int J Mol Sci. 2021. PMID: 34069531 Free PMC article.
-
Okadaic acid induced neurotoxicity: an emerging tool to study Alzheimer's disease pathology.Neurotoxicology. 2013 Jul;37:163-72. doi: 10.1016/j.neuro.2013.05.002. Epub 2013 May 17. Neurotoxicology. 2013. PMID: 23688530
-
Intracerebroventricular administration of okadaic acid induces hippocampal glucose uptake dysfunction and tau phosphorylation.Brain Res Bull. 2016 Jun;124:136-43. doi: 10.1016/j.brainresbull.2016.04.014. Epub 2016 Apr 21. Brain Res Bull. 2016. PMID: 27108544
-
Tauopathy: A common mechanism for neurodegeneration and brain aging.Mech Ageing Dev. 2019 Mar;178:72-79. doi: 10.1016/j.mad.2019.01.007. Epub 2019 Jan 19. Mech Ageing Dev. 2019. PMID: 30668956 Free PMC article. Review.
-
Mechanisms of tau self-aggregation and neurotoxicity.Curr Alzheimer Res. 2011 Sep;8(6):608-14. doi: 10.2174/156720511796717258. Curr Alzheimer Res. 2011. PMID: 21605046 Review.
Cited by
-
The insect, Galleria mellonella, is a compatible model for evaluating the toxicology of okadaic acid.Cell Biol Toxicol. 2019 Jun;35(3):219-232. doi: 10.1007/s10565-018-09448-2. Epub 2018 Nov 13. Cell Biol Toxicol. 2019. PMID: 30426330 Free PMC article.
-
Okadaic acid induces tau phosphorylation in SH-SY5Y cells in an estrogen-preventable manner.Brain Res. 2010 Jul 23;1345:176-81. doi: 10.1016/j.brainres.2010.04.074. Epub 2010 May 7. Brain Res. 2010. PMID: 20457142 Free PMC article.
-
Novel Peptidomic Approach for Identification of Low and High Molecular Weight Tauopathy Peptides Following Calpain Digestion, and Primary Culture Neurotoxic Challenges.Int J Mol Sci. 2019 Oct 21;20(20):5213. doi: 10.3390/ijms20205213. Int J Mol Sci. 2019. PMID: 31640160 Free PMC article.
-
Melatonin Rescues the Dendrite Collapse Induced by the Pro-Oxidant Toxin Okadaic Acid in Organotypic Cultures of Rat Hilar Hippocampus.Molecules. 2020 Nov 25;25(23):5508. doi: 10.3390/molecules25235508. Molecules. 2020. PMID: 33255515 Free PMC article.
-
Quercetin Protects against Okadaic Acid-Induced Injury via MAPK and PI3K/Akt/GSK3β Signaling Pathways in HT22 Hippocampal Neurons.PLoS One. 2016 Apr 6;11(4):e0152371. doi: 10.1371/journal.pone.0152371. eCollection 2016. PLoS One. 2016. PMID: 27050422 Free PMC article.
References
-
- Abrahams S, Pickering A, Polkey CE, Morris RG. Spatial memory deficits in patients with unilateral damage to the right hippocampal formation. Neuropsychologia. 1997;35:11–24. - PubMed
-
- Ahlijanian MK, Barrezueta NX, Williams RD, Jakowski A, Kowsz KP, McCarthy S, Coskran T, Carlo A, Seymour PA, Burkhardt JE, Nelson RB, McNeish JD. Hyperphosphorylated tau and neurofilament and cytoskeletal disruptions in mice overexpressing human p25, an activator of cdk5. Proc Natl Acad Sci U S A. 2000;97:2910–2915. - PMC - PubMed
-
- Alvarez-de-la-Rosa M, Silva I, Nilsen J, Perez MM, Garcia-Segura LM, Avila J, Naftolin F. Estradiol prevents neural tau hyperphosphorylation characteristic of Alzheimer’s disease. Ann N Y Acad Sci. 2005;1052:210–224. - PubMed
-
- Arendt T, Holzer M, Bruckner MK, Janke C, Gartner U. The use of okadaic acid in vivo and the induction of molecular changes typical for Alzheimer’s disease. Neuroscience. 1998a;85:1337–1340. - PubMed
-
- Arendt T, Holzer M, Fruth R, Bruckner MK, Gartner U. Phosphorylation of tau, abeta-formation, and apoptosis after in vivo inhibition of PP-1 and PP-2A. Neurobiol Aging. 1998b;19:3–13. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

