Partial rescue of the Tbx1 mutant heart phenotype by Fgf8: genetic evidence of impaired tissue response to Fgf8
- PMID: 20807544
- PMCID: PMC2981862
- DOI: 10.1016/j.yjmcc.2010.08.023
Partial rescue of the Tbx1 mutant heart phenotype by Fgf8: genetic evidence of impaired tissue response to Fgf8
Abstract
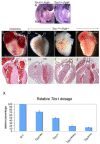
Tbx1 is the candidate gene of DiGeorge syndrome and is required in humans and mice for the development of the cardiac outflow tract (OFT) and aortic arch arteries. Loss of function mutants present with reduced cell proliferation and premature differentiation of cardiac progenitor cells of the second heart field (SHF). Tbx1 regulates Fgf8 expression hence the hypothesis that the proliferation impairment may contribute to the heart phenotype of mutants. Here we show that forced Fgf8 expression modifies and partially rescues the OFT septation defects of Tbx1 mutants but only if there is some residual expression of Tbx1. This genetic experiment suggests that Tbx1, directly or indirectly, affects tissue response to Fgf8. Indeed, Tbx1(-/-) mouse embryonic fibroblasts were unable to respond to Fgf8 added to the culture media and showed defective response of Erk1/2 and Rsk1. Our data suggest a coordinated pathway modulating Fgf8 ligand expression and tissue response to it in the SHF.
Copyright © 2010 Elsevier Ltd. All rights reserved.
Figures

References
-
- Prall OW, Elliott DA, Harvey RP. Developmental paradigms in heart disease: insights from tinman. Ann Med. 2002;34:148–56. - PubMed
-
- Prall OW, Menon MK, Solloway MJ, Watanabe Y, Zaffran S, Bajolle F, Biben C, McBride JJ, Robertson BR, Chaulet H, Stennard FA, Wise N, Schaft D, Wolstein O, Furtado MB, Shiratori H, Chien KR, Hamada H, Black BL, Saga Y, Robertson EJ, Buckingham ME, Harvey RP. An Nkx2-5/Bmp2/Smad1 negative feedback loop controls heart progenitor specification and proliferation. Cell. 2007;128:947–59. - PMC - PubMed
-
- Vitelli F, Taddei I, Morishima M, Meyers EN, Lindsay EA, Baldini A. A genetic link between Tbx1 and Fibroblast Growth Factor signaling. Development. 2002;129:4605–4611. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

