An oxidative environment promotes growth of Mycobacterium abscessus
- PMID: 20807564
- PMCID: PMC2970643
- DOI: 10.1016/j.freeradbiomed.2010.08.026
An oxidative environment promotes growth of Mycobacterium abscessus
Abstract
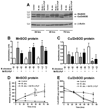
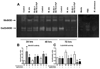
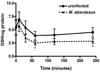
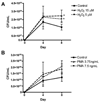
Mycobacterium abscessus infections, particularly those causing chronic lung diseases, are becoming more prevalent worldwide. M. abscessus infections are difficult to treat because of antibiotic resistance. Thus, new treatment options are urgently needed. M. abscessus is an intracellular pathogen that primarily infects macrophages and fibroblasts. Because this bacterium has only recently been identified as a separate species, very little is known about M. abscessus-host interactions and how M. abscessus growth is regulated. Oxidative stress has long been shown to inhibit the growth of bacterial organisms. However, some intracellular bacteria, such as Mycobacterium tuberculosis, grow well in oxidizing environments. In this study, we show that M. abscessus infection causes the host cell environment to become more oxidizing. Furthermore, we show that a more oxidizing environment leads to enhanced growth of M. abscessus inside macrophages. In the presence of antioxidants, MnTE-2-PyP (chemical name: manganese(II) meso-tetrakis-(N-methylpyridinium-2-yl) porphyrin) or N-acetyl-l-cysteine, M. abscessus growth is inhibited. These results lead us to postulate that antioxidants may aid in the treatment of M. abscessus infections.
Copyright © 2010 Elsevier Inc. All rights reserved.
Figures




Similar articles
-
Mycobacterium abscessus induces a limited pattern of neutrophil activation that promotes pathogen survival.PLoS One. 2013;8(2):e57402. doi: 10.1371/journal.pone.0057402. Epub 2013 Feb 25. PLoS One. 2013. PMID: 23451220 Free PMC article.
-
GM-CSF knockout mice for preclinical testing of agents with antimicrobial activity against Mycobacterium abscessus.J Antimicrob Chemother. 2014 Apr;69(4):1057-64. doi: 10.1093/jac/dkt451. Epub 2013 Nov 11. J Antimicrob Chemother. 2014. PMID: 24222613
-
The antioxidant mimetic, MnTE-2-PyP, reduces intracellular growth of Mycobacterium abscessus.Am J Respir Cell Mol Biol. 2009 Aug;41(2):170-8. doi: 10.1165/rcmb.2008-0138OC. Epub 2008 Dec 18. Am J Respir Cell Mol Biol. 2009. PMID: 19097985 Free PMC article.
-
Mycobacterium abscessus: a new antibiotic nightmare.J Antimicrob Chemother. 2012 Apr;67(4):810-8. doi: 10.1093/jac/dkr578. Epub 2012 Jan 30. J Antimicrob Chemother. 2012. PMID: 22290346 Review.
-
Preclinical murine models to study lung infection with Mycobacterium abscessus complex.Tuberculosis (Edinb). 2023 Jan;138:102301. doi: 10.1016/j.tube.2022.102301. Epub 2022 Dec 29. Tuberculosis (Edinb). 2023. PMID: 36603391 Review.
Cited by
-
The Role of NRF2 in Mycobacterial Infection.Antioxidants (Basel). 2021 Nov 23;10(12):1861. doi: 10.3390/antiox10121861. Antioxidants (Basel). 2021. PMID: 34942964 Free PMC article. Review.
-
Superoxide Generation and Its Involvement in the Growth of Mycobacterium smegmatis.Front Microbiol. 2017 Jan 30;8:105. doi: 10.3389/fmicb.2017.00105. eCollection 2017. Front Microbiol. 2017. PMID: 28194149 Free PMC article.
-
Adding Another Piece to the Puzzle of Why NTM Infections Are Relatively Uncommon despite Their Ubiquitous Nature.mBio. 2021 Apr 20;12(2):e03577-20. doi: 10.1128/mBio.03577-20. mBio. 2021. PMID: 33879587 Free PMC article.
-
Mycobacterium abscessus Clearance by Neutrophils Is Independent of Autophagy.Infect Immun. 2020 Jul 21;88(8):e00024-20. doi: 10.1128/IAI.00024-20. Print 2020 Jul 21. Infect Immun. 2020. PMID: 32423916 Free PMC article.
-
Caspase-independent apoptosis in infected macrophages triggered by sulforaphane via Nrf2/p38 signaling pathways.Cell Death Discov. 2015 Aug 24;1:15022. doi: 10.1038/cddiscovery.2015.22. eCollection 2015. Cell Death Discov. 2015. PMID: 27551455 Free PMC article.
References
-
- Petrini B. Mycobacterium abscessus: an emerging rapid-growing potential pathogen. Apmis. 2006;114:319–328. - PubMed
-
- Cassidy PM, Hedberg K, Saulson A, McNelly E, Winthrop KL. Nontuberculous mycobacterial disease prevalence and risk factors: a changing epidemiology. Clin Infect Dis. 2009;49:e124–e129. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

