An integrated approach for the rational design of nanovectors for biomedical imaging and therapy
- PMID: 20807601
- PMCID: PMC3998831
- DOI: 10.1016/S0065-2660(10)69009-8
An integrated approach for the rational design of nanovectors for biomedical imaging and therapy
Abstract
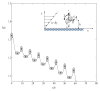
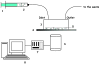
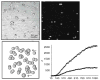
The use of nanoparticles for the early detection, cure, and imaging of diseases has been proved already to have a colossal potential in different biomedical fields, such as oncology and cardiology. A broad spectrum of nanoparticles are currently under development, exhibiting differences in (i) size, ranging from few tens of nanometers to few microns; (ii) shape, from the classical spherical beads to discoidal, hemispherical, cylindrical, and conical; (iii) surface functionalization, with a wide range of electrostatic charges and biomolecule conjugations. Clearly, the library of nanoparticles generated by combining all possible sizes, shapes, and surface physicochemical properties is enormous. With such a complex scenario, an integrated approach is here proposed and described for the rational design of nanoparticle systems (nanovectors) for the intravascular delivery of therapeutic and imaging contrast agents. The proposed integrated approach combines multiscale/multiphysics mathematical models with in vitro assays and in vivo intravital microscopy (IVM) experiments and aims at identifying the optimal combination of size, shape, and surface properties that maximize the nanovectors localization within the diseased microvasculature.
Copyright (c) 2010 Elsevier Inc. All rights reserved.
Figures







References
-
- Adams GP, Schier R, McCall AM, Simmons HH, Horak EM, Alpaugh RK, Marks JD, Weiner LM. High affinity restricts the localization and tumor penetration of single-chain fv antibody molecules. Cancer Res. 2001;61:4750. - PubMed
-
- Allen TM. Ligand-targeted therapeutics in anticancer therapy Nat. Rev Drug Discov. 2002;2:750. - PubMed
-
- Allen TM. Ligand-targeted therapeutics in anticancer therapy. Nat Rev Drug Discov. 2002;2:750. - PubMed
-
- Allen TM, Ahmad I, Lopes de Menezes DE, Moase EH. Immunoliposome-mediated targeting of anti-cancer drugs in vivo. Biochem Soc Trans. 1995;23:1073–1079. - PubMed
-
- Allen TM, Sapra P, Moase E, Moreira J, Iden D. Adventures in targeting. J Liposome Res. 2002;12:5–12. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

