VIVID interacts with the WHITE COLLAR complex and FREQUENCY-interacting RNA helicase to alter light and clock responses in Neurospora
- PMID: 20807745
- PMCID: PMC2944716
- DOI: 10.1073/pnas.1009474107
VIVID interacts with the WHITE COLLAR complex and FREQUENCY-interacting RNA helicase to alter light and clock responses in Neurospora
Abstract
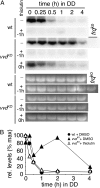
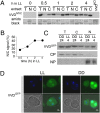
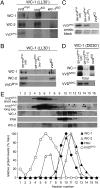
The photoreceptor and PAS/LOV protein VIVID (VVD) modulates blue-light signaling and influences light and temperature responses of the circadian clock in Neurospora crassa. One of the main actions of VVD on the circadian clock is to influence circadian clock phase by regulating levels of the transcripts encoded by the central clock gene frequency (frq). How this regulation is achieved is unknown. Here we show that VVD interacts with complexes central for circadian clock and blue-light signaling, namely the WHITE-COLLAR complex (WCC) and FREQUENCY-interacting RNA helicase (FRH), a component that complexes with FRQ to mediate negative feedback control in Neurospora. VVD interacts with FRH in the absence of WCC and FRQ but does not seem to control the exosome-mediated negative feedback loop. Instead, VVD acts to modulate the transcriptional activity of the WCC.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

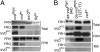
Similar articles
-
FRQ-interacting RNA helicase mediates negative and positive feedback in the Neurospora circadian clock.Genetics. 2010 Feb;184(2):351-61. doi: 10.1534/genetics.109.111393. Epub 2009 Nov 30. Genetics. 2010. PMID: 19948888 Free PMC article.
-
Regulation of the Neurospora circadian clock by an RNA helicase.Genes Dev. 2005 Jan 15;19(2):234-41. doi: 10.1101/gad.1266805. Epub 2004 Dec 29. Genes Dev. 2005. PMID: 15625191 Free PMC article.
-
Physical interaction between VIVID and white collar complex regulates photoadaptation in Neurospora.Proc Natl Acad Sci U S A. 2010 Sep 21;107(38):16715-20. doi: 10.1073/pnas.1011190107. Epub 2010 Aug 23. Proc Natl Acad Sci U S A. 2010. PMID: 20733070 Free PMC article.
-
The molecular workings of the Neurospora biological clock.Novartis Found Symp. 2003;253:184-98; discussion 102-9, 198-202, 281-4. Novartis Found Symp. 2003. PMID: 14712922 Review.
-
A circadian clock in Neurospora: how genes and proteins cooperate to produce a sustained, entrainable, and compensated biological oscillator with a period of about a day.Cold Spring Harb Symp Quant Biol. 2007;72:57-68. doi: 10.1101/sqb.2007.72.072. Cold Spring Harb Symp Quant Biol. 2007. PMID: 18522516 Free PMC article. Review.
Cited by
-
Inhibitor of DNA binding 2 (Id2) Regulates Photic Entrainment Responses in Mice: Differential Responses of the Id2-/- Mouse Circadian System Are Dependent on Circadian Phase and on Duration and Intensity of Light.J Biol Rhythms. 2020 Dec;35(6):555-575. doi: 10.1177/0748730420957504. Epub 2020 Sep 28. J Biol Rhythms. 2020. PMID: 32981454 Free PMC article.
-
Light-induced subunit dissociation by a light-oxygen-voltage domain photoreceptor from Rhodobacter sphaeroides.Biochemistry. 2013 Jan 15;52(2):378-91. doi: 10.1021/bi3015373. Epub 2013 Jan 3. Biochemistry. 2013. PMID: 23252338 Free PMC article.
-
Functional and topological diversity of LOV domain photoreceptors.Proc Natl Acad Sci U S A. 2016 Mar 15;113(11):E1442-51. doi: 10.1073/pnas.1509428113. Epub 2016 Feb 29. Proc Natl Acad Sci U S A. 2016. PMID: 26929367 Free PMC article.
-
Comprehensive modelling of the Neurospora circadian clock and its temperature compensation.PLoS Comput Biol. 2012;8(3):e1002437. doi: 10.1371/journal.pcbi.1002437. Epub 2012 Mar 29. PLoS Comput Biol. 2012. PMID: 22496627 Free PMC article.
-
ENVOY is a major determinant in regulation of sexual development in Hypocrea jecorina (Trichoderma reesei).Eukaryot Cell. 2012 Jul;11(7):885-95. doi: 10.1128/EC.05321-11. Epub 2012 May 11. Eukaryot Cell. 2012. PMID: 22581525 Free PMC article.
References
-
- Dunlap JC, Loros JJ, DeCoursey PJ. Chronobiology. Sunderland. MA: Sinauer Associates, Inc.; 2004.
-
- Johnson CH, Elliott JA, Foster R. Entrainment of circadian programs. Chronobiol Int. 2003;20:741–774. - PubMed
-
- Price-Lloyd N, Elvin M, Heintzen C. Synchronizing the Neurospora crassa circadian clock with the rhythmic environment. Biochem Soc Trans. 2005;33:949–952. - PubMed
-
- Crosthwaite SK, Dunlap JC, Loros JJ. Neurospora wc-1 and wc-2: Transcription, photoresponses, and the origins of circadian rhythmicity. Science. 1997;276:763–769. - PubMed
-
- Gehring W, Rosbash M. The coevolution of blue-light photoreception and circadian rhythms. J Mol Evol. 2003;57(Suppl 1):S286–S289. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

