N-terminal domain of myelin basic protein inhibits amyloid beta-protein fibril assembly
- PMID: 20807757
- PMCID: PMC2975183
- DOI: 10.1074/jbc.M110.169599
N-terminal domain of myelin basic protein inhibits amyloid beta-protein fibril assembly
Abstract
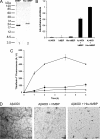
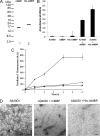
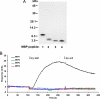
Accumulation of amyloid β-protein (Aβ) into brain parenchymal plaques and the cerebral vasculature is a pathological feature of Alzheimer disease and related disorders. Aβ peptides readily form β-sheet-containing oligomers and fibrils. Previously, we reported a strong interaction between myelin basic protein (MBP) and Aβ peptides that resulted in potent inhibition of fibril assembly (Hoos, M. D., Ahmed, M., Smith, S. O., and Van Nostrand, W. E. (2007) J. Biol. Chem. 282, 9952-9961; Hoos, M. D., Ahmed, M., Smith, S. O., and Van Nostrand, W. E. (2009) Biochemistry 48, 4720-4727). MBP is recognized as a highly post-translationally modified protein. In the present study, we demonstrate that human MBP purified from either brain or a bacterial recombinant expression system comparably bound to Aβ and inhibited Aβ fibril assembly indicating that post-translational modifications are not required for this activity. We also show that purified mouse brain MBP and recombinantly expressed mouse MBP similarly inhibited Aβ fibril formation. Through a combination of biochemical and ultrastructural techniques, we demonstrate that the binding site for Aβ is located in the N-terminal 64 amino acids of MBP and that a stable peptide (MBP1) comprising these residues was sufficient to inhibit Aβ fibrillogenesis. Under conditions comparable with those used for Aβ, the fibrillar assembly of amylin, another amyloidogenic peptide, was not inhibited by MBP1, although MBP1 still bound to it. This observation suggests that the potent inhibitory effect of MBP on fibril formation is not general to amyloidogenic peptides. Finally, MBP1 could prevent the cytotoxic effects of Aβ in primary cortical neurons. Our findings suggest that inhibition of Aβ fibril assembly by MBP, mediated through its N-terminal domain, could play a role in influencing amyloid formation in Alzheimer disease brain and corresponding mouse models.
Figures
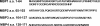

Similar articles
-
Fine mapping of the amyloid β-protein binding site on myelin basic protein.Biochemistry. 2013 Apr 16;52(15):2565-73. doi: 10.1021/bi4001936. Epub 2013 Apr 4. Biochemistry. 2013. PMID: 23510371 Free PMC article.
-
Degradation of amyloid beta protein by purified myelin basic protein.J Biol Chem. 2009 Oct 16;284(42):28917-25. doi: 10.1074/jbc.M109.050856. Epub 2009 Aug 19. J Biol Chem. 2009. PMID: 19692707 Free PMC article.
-
Myelin basic protein binds to and inhibits the fibrillar assembly of Abeta42 in vitro.Biochemistry. 2009 Jun 9;48(22):4720-7. doi: 10.1021/bi900037s. Biochemistry. 2009. PMID: 19385666 Free PMC article.
-
New Mechanism of Amyloid Fibril Formation.Curr Protein Pept Sci. 2019;20(6):630-640. doi: 10.2174/1389203720666190125160937. Curr Protein Pept Sci. 2019. PMID: 30686252 Review.
-
Solid-state NMR as a method to reveal structure and membrane-interaction of amyloidogenic proteins and peptides.Biochim Biophys Acta. 2007 Aug;1768(8):1900-12. doi: 10.1016/j.bbamem.2007.03.025. Epub 2007 Apr 5. Biochim Biophys Acta. 2007. PMID: 17524351 Review.
Cited by
-
Gene Expression of Quaking in Sporadic Alzheimer's Disease Patients is Both Upregulated and Related to Expression Levels of Genes Involved in Amyloid Plaque and Neurofibrillary Tangle Formation.J Alzheimers Dis. 2016 May 6;53(1):209-19. doi: 10.3233/JAD-160160. J Alzheimers Dis. 2016. PMID: 27163826 Free PMC article.
-
The formation of tau pore-like structures is prevalent and cell specific: possible implications for the disease phenotypes.Acta Neuropathol Commun. 2014 May 29;2:56. doi: 10.1186/2051-5960-2-56. Acta Neuropathol Commun. 2014. PMID: 24887264 Free PMC article.
-
Oral delivery of bioencapsulated proteins across blood-brain and blood-retinal barriers.Mol Ther. 2014 Mar;22(3):535-546. doi: 10.1038/mt.2013.273. Epub 2013 Dec 6. Mol Ther. 2014. PMID: 24281246 Free PMC article.
-
Oral Delivery of Protein Drugs Bioencapsulated in Plant Cells.Mol Ther. 2016 Aug;24(8):1342-50. doi: 10.1038/mt.2016.115. Epub 2016 Jun 6. Mol Ther. 2016. PMID: 27378236 Free PMC article. Review.
-
Structured functional domains of myelin basic protein: cross talk between actin polymerization and Ca(2+)-dependent calmodulin interaction.Biophys J. 2011 Sep 7;101(5):1248-56. doi: 10.1016/j.bpj.2011.07.035. Biophys J. 2011. PMID: 21889463 Free PMC article.
References
-
- Selkoe D. J. (2001) Physiol. Rev. 81, 741–766 - PubMed
-
- Kang J., Lemaire H. G., Unterbeck A., Salbaum J. M., Masters C. L., Grzeschik K. H., Multhaup G., Beyreuther K., Müller-Hill B. (1987) Nature 325, 733–736 - PubMed
-
- Goldgaber D., Lerman M. I., McBride O. W., Saffiotti U., Gajdusek D. C. (1987) Science 235, 877–880 - PubMed
-
- Tanzi R. E., Gusella J. F., Watkins P. C., Bruns G. A., St George-Hyslop P., Van Keuren M. L., Patterson D., Pagan S., Kurnit D. M., Neve R. L. (1987) Science 235, 880–884 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

