Regulated secretion of acid sphingomyelinase: implications for selectivity of ceramide formation
- PMID: 20807762
- PMCID: PMC2975195
- DOI: 10.1074/jbc.M110.125609
Regulated secretion of acid sphingomyelinase: implications for selectivity of ceramide formation
Abstract
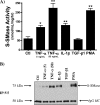
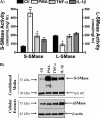
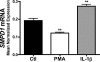
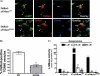
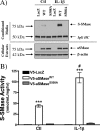
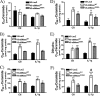
The acid sphingomyelinase (aSMase) gene gives rise to two distinct enzymes, lysosomal sphingomyelinase (L-SMase) and secretory sphingomyelinase (S-SMase), via differential trafficking of a common protein precursor. However, the regulation of S-SMase and its role in cytokine-induced ceramide formation remain ill defined. To determine the role of S-SMase in cellular sphingolipid metabolism, MCF7 breast carcinoma cells stably transfected with V5-aSMase(WT) were treated with inflammatory cytokines. Interleukin-1β and tumor necrosis factor-α induced a time- and dose-dependent increase in S-SMase secretion and activity, coincident with selective elevations in cellular C(16)-ceramide. To establish a role for S-SMase, we utilized a mutant of aSMase (S508A) that is shown to retain L-SMase activity, but is defective in secretion. MCF7 expressing V5-aSMase(WT) exhibited increased S-SMase and L-SMase activity, as well as elevated cellular levels of specific long-chain and very long-chain ceramide species relative to vector control MCF7. Interestingly, elevated levels of only certain very long-chain ceramides were evident in V5-aSMase(S508A) MCF7. Secretion of the S508A mutant was also defective in response to IL-1β, as was the regulated generation of C(16)-ceramide. Taken together, these data support a crucial role for Ser(508) in the regulation of S-SMase secretion, and they suggest distinct metabolic roles for S-SMase and L-SMase.
Figures




Similar articles
-
Regulation of CC ligand 5/RANTES by acid sphingomyelinase and acid ceramidase.J Biol Chem. 2011 Apr 15;286(15):13292-303. doi: 10.1074/jbc.M110.163378. Epub 2011 Feb 18. J Biol Chem. 2011. PMID: 21335555 Free PMC article.
-
A novel mechanism of lysosomal acid sphingomyelinase maturation: requirement for carboxyl-terminal proteolytic processing.J Biol Chem. 2011 Feb 4;286(5):3777-88. doi: 10.1074/jbc.M110.155234. Epub 2010 Nov 22. J Biol Chem. 2011. PMID: 21098024 Free PMC article.
-
Ceramide induces aSMase expression: implications for oxLDL-induced apoptosis.FASEB J. 2001 Mar;15(3):807-14. doi: 10.1096/fj.15.3.807. FASEB J. 2001. PMID: 11259399
-
Secretory sphingomyelinase.Chem Phys Lipids. 1999 Nov;102(1-2):123-30. doi: 10.1016/s0009-3084(99)00080-8. Chem Phys Lipids. 1999. PMID: 11001566 Review.
-
The acid sphingomyelinase/ceramide pathway: biomedical significance and mechanisms of regulation.Curr Mol Med. 2010 Jul;10(5):454-66. doi: 10.2174/156652410791608225. Curr Mol Med. 2010. PMID: 20540705 Review.
Cited by
-
Evaluation of the role of secretory sphingomyelinase and bioactive sphingolipids as biomarkers in hemophagocytic lymphohistiocytosis.Am J Hematol. 2013 Nov;88(11):E265-72. doi: 10.1002/ajh.23535. Epub 2013 Aug 30. Am J Hematol. 2013. PMID: 23828274 Free PMC article.
-
Ceramide signaling in the gut.Mol Cell Endocrinol. 2022 Mar 15;544:111554. doi: 10.1016/j.mce.2022.111554. Epub 2022 Jan 5. Mol Cell Endocrinol. 2022. PMID: 34998898 Free PMC article. Review.
-
Sphingomyelinase decreases transepithelial anion secretion in airway epithelial cells in part by inhibiting CFTR-mediated apical conductance.Physiol Rep. 2021 Aug;9(15):e14928. doi: 10.14814/phy2.14928. Physiol Rep. 2021. PMID: 34382377 Free PMC article.
-
Inhibiting glucosylceramide synthase exacerbates cisplatin-induced acute kidney injury.J Lipid Res. 2017 Jul;58(7):1439-1452. doi: 10.1194/jlr.M076745. Epub 2017 May 10. J Lipid Res. 2017. PMID: 28490444 Free PMC article.
-
Differential regulation of acid sphingomyelinase in macrophages stimulated with oxidized low-density lipoprotein (LDL) and oxidized LDL immune complexes: role in phagocytosis and cytokine release.Immunology. 2012 May;136(1):30-45. doi: 10.1111/j.1365-2567.2012.03552.x. Immunology. 2012. PMID: 22236141 Free PMC article.
References
-
- Hannun Y. A., Obeid L. M. (2008) Nat. Rev. Mol. Cell Biol. 9, 139–150 - PubMed
-
- Gulbins E., Li P. L. (2006) Am. J. Physiol. Regul. Integr. Comp. Physiol. 290, R11–26 - PubMed
-
- Pettus B. J., Chalfant C. E., Hannun Y. A. (2004) Curr. Mol. Med. 4, 405–418 - PubMed
-
- Ogretmen B., Hannun Y. A. (2004) Nat. Rev. Cancer. 4, 604–616 - PubMed
-
- Goñi F. M., Alonso A. (2002) FEBS Lett. 531, 38–46 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

