Oligomeric amyloid-{beta} inhibits the proteolytic conversion of brain-derived neurotrophic factor (BDNF), AMPA receptor trafficking, and classical conditioning
- PMID: 20807770
- PMCID: PMC2966086
- DOI: 10.1074/jbc.M110.150821
Oligomeric amyloid-{beta} inhibits the proteolytic conversion of brain-derived neurotrophic factor (BDNF), AMPA receptor trafficking, and classical conditioning
Abstract
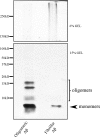
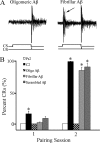
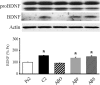
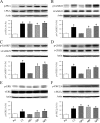
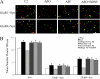
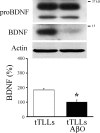
Amyloid-β (Aβ) peptide is thought to have a significant role in the progressive memory loss observed in patients with Alzheimer disease and inhibits synaptic plasticity in animal models of learning. We previously demonstrated that brain-derived neurotrophic factor (BDNF) is critical for synaptic AMPA receptor delivery in an in vitro model of eyeblink classical conditioning. Here, we report that acquisition of conditioned responses was significantly attenuated by bath application of oligomeric (200 nm), but not fibrillar, Aβ peptide. Western blotting revealed that BDNF protein expression during conditioning is significantly reduced by treatment with oligomeric Aβ, as were phosphorylation levels of cAMP-response element-binding protein (CREB), Ca(2+)/calmodulin-dependent protein kinase II (CaMKII), Ca(2+)/calmodulin-dependent protein kinase IV (CaMKIV), and ERK. However, levels of PKA and PKCζ/λ were unaffected, as was PDK-1. Protein localization studies using confocal imaging indicate that oligomeric Aβ, but not fibrillar or scrambled forms, suppresses colocalization of GluR1 and GluR4 AMPA receptor subunits with synaptophysin, indicating that trafficking of these subunits to synapses during the conditioning procedure is blocked. In contrast, coapplication of BDNF with oligomeric Aβ significantly reversed these findings. Interestingly, a tolloid-like metalloproteinase in turtle, tTLLs (turtle tolloid-like protein), which normally processes the precursor proBDNF into mature BDNF, was found to degrade oligomeric Aβ into small fragments. These data suggest that an Aβ-induced reduction in BDNF, perhaps due to interference in the proteolytic conversion of proBDNF to BDNF, results in inhibition of synaptic AMPA receptor delivery and suppression of the acquisition of conditioning.
Figures


Similar articles
-
PKA has a critical role in synaptic delivery of GluR1- and GluR4-containing AMPARs during initial stages of acquisition of in vitro classical conditioning.J Neurophysiol. 2009 May;101(5):2539-49. doi: 10.1152/jn.91282.2008. Epub 2009 Mar 4. J Neurophysiol. 2009. PMID: 19261706 Free PMC article.
-
Cleavage of proBDNF to BDNF by a tolloid-like metalloproteinase is required for acquisition of in vitro eyeblink classical conditioning.J Neurosci. 2009 Nov 25;29(47):14956-64. doi: 10.1523/JNEUROSCI.3649-09.2009. J Neurosci. 2009. PMID: 19940191 Free PMC article.
-
BDNF-induced synaptic delivery of AMPAR subunits is differentially dependent on NMDA receptors and requires ERK.Neurobiol Learn Mem. 2009 Mar;91(3):243-9. doi: 10.1016/j.nlm.2008.10.002. Epub 2008 Nov 17. Neurobiol Learn Mem. 2009. PMID: 18977306 Free PMC article.
-
Regulation of AMPA receptors by phosphorylation.Neurochem Res. 2000 Oct;25(9-10):1245-55. doi: 10.1023/a:1007644128886. Neurochem Res. 2000. PMID: 11059799 Review.
-
Synaptic Mechanisms of Delay Eyeblink Classical Conditioning: AMPAR Trafficking and Gene Regulation in an In Vitro Model.Mol Neurobiol. 2023 Dec;60(12):7088-7103. doi: 10.1007/s12035-023-03528-z. Epub 2023 Aug 2. Mol Neurobiol. 2023. PMID: 37531025 Review.
Cited by
-
Characterization of Aldh2 (-/-) mice as an age-related model of cognitive impairment and Alzheimer's disease.Mol Brain. 2015 Apr 25;8:27. doi: 10.1186/s13041-015-0117-y. Mol Brain. 2015. PMID: 25910195 Free PMC article.
-
Converged avenues: depression and Alzheimer's disease- shared pathophysiology and novel therapeutics.Mol Biol Rep. 2024 Jan 28;51(1):225. doi: 10.1007/s11033-023-09170-1. Mol Biol Rep. 2024. PMID: 38281208 Review.
-
The neuroprotective effects of glucagon-like peptide 1 in Alzheimer's and Parkinson's disease: An in-depth review.Front Neurosci. 2022 Sep 1;16:970925. doi: 10.3389/fnins.2022.970925. eCollection 2022. Front Neurosci. 2022. PMID: 36117625 Free PMC article. Review.
-
An Interaction between Brain-Derived Neurotrophic Factor and Stress-Related Glucocorticoids in the Pathophysiology of Alzheimer's Disease.Int J Mol Sci. 2024 Jan 27;25(3):1596. doi: 10.3390/ijms25031596. Int J Mol Sci. 2024. PMID: 38338875 Free PMC article. Review.
-
Effects of Pretreatment With Coenzyme Q10 (CoQ10) and High-Intensity Interval Training (HIIT) on FNDC5, Irisin, and BDNF Levels, and Amyloid-Beta (Aβ) Plaque Formation in the Hippocampus of Aβ-Induced Alzheimer's Disease Rats.CNS Neurosci Ther. 2025 Feb;31(2):e70221. doi: 10.1111/cns.70221. CNS Neurosci Ther. 2025. PMID: 39957598 Free PMC article.
References
-
- Almeida C. G., Tampellini D., Takahashi R. H., Greengard P., Lin M. T., Snyder E. M., Gouras G. K. (2005) Neurobiol. Dis. 20, 187–198 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

