Orexin neurons are indispensable for stress-induced thermogenesis in mice
- PMID: 20807795
- PMCID: PMC3002445
- DOI: 10.1113/jphysiol.2010.195099
Orexin neurons are indispensable for stress-induced thermogenesis in mice
Abstract
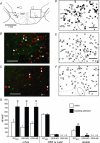
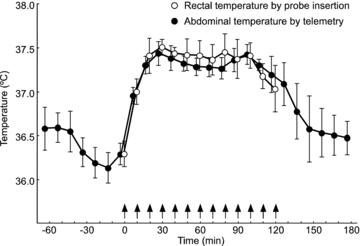
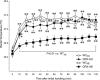
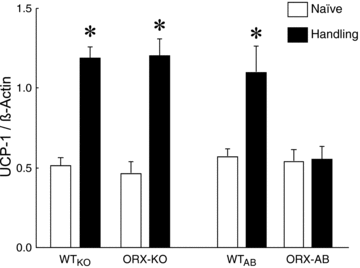
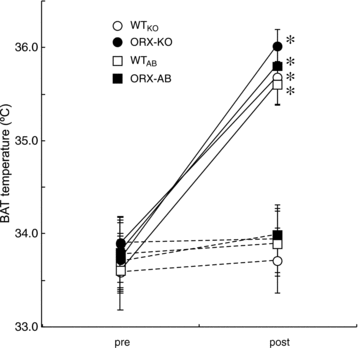
Orexin neurons contribute to cardiovascular, respiratory and analgesic components of the fight-or-flight response against stressors. Here, we examined whether the same is true for stress-induced hyperthermia. We used prepro-orexin knockout mice (ORX-KO) and orexin neuron-ablated mice (ORX-AB) in which the latter lack not only orexin, but also other putative neurotransmitter/modulators contained in the orexin neurons. In response to repetitive insertion of a temperature probe into their rectum (handling stress), ORX-KO mice showed a normal temperature change as compared to that of wild-type littermates (WT) while ORX-AB showed an attenuated response. Stress-induced expression of uncoupling protein-1, a key molecule in non-shivering thermogenesis in the brown adipose tissue (BAT), was also blunted in ORX-AB but not in ORX-KO. When the BAT was directly activated by a β3 adrenergic agonist, there was no difference in the resultant BAT temperature among the groups, indicating that BAT per se was normal in ORX-AB. In WT and ORX-KO, handling stress activated orexin neurons (as revealed by increased expression of c-Fos) and the resultant hyperthermia was largely blunted by pre-treatment with a β3 antagonist. This observation further supports the notion that attenuated stress-induced hyperthermia in ORX-AB mice was caused by a loss of orexin neurons and abnormal BAT regulation. This study pointed out, for the first time, the possible importance of co-existent neurotransmitter/modulators in the orexin neurons for stress-induced hyperthermia and the importance of integrity of the orexin neurons for full expression of multiple facets of the fight-or-flight response.
Figures
Comment in
-
The peptide or the neuron?J Physiol. 2010 Nov 1;588(Pt 21):4067-8. doi: 10.1113/jphysiol.2010.199745. J Physiol. 2010. PMID: 21037314 Free PMC article. No abstract available.
References
-
- Abrahamson EE, Leak RK, Moore RY. The suprachiasmatic nucleus projects to posterior hypothalamic arousal systems. Neuroreport. 2001;12:435–440. - PubMed
-
- Berthoud H-R, Patterson LM, Sutton GM, Morrison C, Zheng H. Orexin inputs to caudal raphé neurons involved in thermal, cardiovascular, and gastrointestinal regulation. Histochem Cell Biol. 2005;123:147–156. - PubMed
-
- Bouwknecht JA, Olivier B, Paylor RE. The stress-induced hyperthermia paradigm as a physiological animal model for anxiety: a review of pharmacological and genetic studies in the mouse. Neurosci Biobehav Rev. 2007;31:41–59. - PubMed
-
- Cannon B, Nedergaard J. Brown adipose tissue: function and physiological significance. Physiol Rev. 2004;84:277–359. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

