The recruitment of acetylated and unacetylated tropomyosin to distinct actin polymers permits the discrete regulation of specific myosins in fission yeast
- PMID: 20807799
- PMCID: PMC2939800
- DOI: 10.1242/jcs.069971
The recruitment of acetylated and unacetylated tropomyosin to distinct actin polymers permits the discrete regulation of specific myosins in fission yeast
Abstract
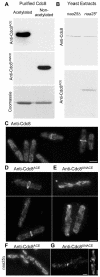
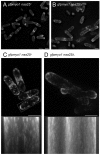
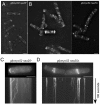
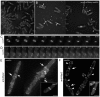
Tropomyosin (Tm) is a conserved dimeric coiled-coil protein, which forms polymers that curl around actin filaments in order to regulate actomyosin function. Acetylation of the Tm N-terminal methionine strengthens end-to-end bonds, which enhances actin binding as well as the ability of Tm to regulate myosin motor activity in both muscle and non-muscle cells. In this study we explore the function of each Tm form within fission yeast cells. Electron microscopy and live cell imaging revealed that acetylated and unacetylated Tm associate with distinct actin structures within the cell, and that each form has a profound effect upon the shape and integrity of the polymeric actin filament. We show that, whereas Tm acetylation is required to regulate the in vivo motility of class II myosins, acetylated Tm had no effect on the motility of class I and V myosins. These findings illustrate a novel Tm-acetylation-state-dependent mechanism for regulating specific actomyosin cytoskeletal interactions.
Figures


References
-
- Attanapola S. L., Alexander C. J., Mulvihill D. P. (2009). Ste20-kinase-dependent TEDS-site phosphorylation modulates the dynamic localisation and endocytic function of the fission yeast class I myosin, Myo1. J. Cell Sci. 122, 3856-3861 - PubMed
-
- Bahler J., Wu J. Q., Longtine M. S., Shah N. G., McKenzie A., 3rd, Steever A. B., Wach A., Philippsen P., Pringle J. R. (1998). Heterologous modules for efficient and versatile PCR-based gene targeting in Schizosaccharomyces pombe. Yeast 14, 943-951 - PubMed
-
- Balasubramanian M. K., Helfman D. M., Hemmingsen S. M. (1992). A new tropomyosin essential for cytokinesis in the fission yeast S. pombe. Nature 360, 84-87 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

