The exocytic gene secA is required for Dictyostelium cell motility and osmoregulation
- PMID: 20807800
- PMCID: PMC2939799
- DOI: 10.1242/jcs.072876
The exocytic gene secA is required for Dictyostelium cell motility and osmoregulation
Abstract
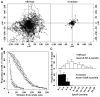
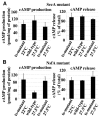
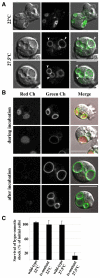
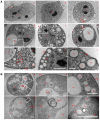
We investigated the link between cell movement and plasma membrane recycling using a fast-acting, temperature-sensitive mutant of the Dictyostelium SecA exocytic protein. Strikingly, most mutant cells become almost paralysed within minutes at the restrictive temperature. However, they can still sense cyclic-AMP (cAMP) gradients and polymerise actin up-gradient, but form only abortive pseudopodia, which cannot expand. They also relay a cAMP signal normally, suggesting that cAMP is released by a non-exocytic mechanism. To investigate why SecA is required for motility, we examined membrane trafficking in the mutant. Plasma membrane circulation is rapidly inhibited at the restrictive temperature and the cells acquire a prominent vesicle. Organelle-specific markers show that this is an undischarged contractile vacuole, and we found the cells are correspondingly osmo-sensitive. Electron microscopy shows that many smaller vesicles, probably originating from the plasma membrane, also accumulate at the restrictive temperature. Consistent with this, the surface area of mutant cells shrinks. We suggest that SecA mutant cells cannot move at the restrictive temperature because their block in exocytosis results in a net uptake of plasma membrane, reducing its area, and so restricting pseudopodial expansion. This demonstrates the importance of proper surface area regulation in cell movement.
Figures



Similar articles
-
Sensitization of Dictyostelium chemotaxis by phosphoinositide-3-kinase-mediated self-organizing signalling patches.J Cell Sci. 2004 Jun 15;117(Pt 14):2925-35. doi: 10.1242/jcs.01143. Epub 2004 May 25. J Cell Sci. 2004. PMID: 15161938
-
Intracellular role of adenylyl cyclase in regulation of lateral pseudopod formation during Dictyostelium chemotaxis.Eukaryot Cell. 2005 Apr;4(4):775-86. doi: 10.1128/EC.4.4.775-786.2005. Eukaryot Cell. 2005. PMID: 15821137 Free PMC article.
-
RasC plays a role in transduction of temporal gradient information in the cyclic-AMP wave of Dictyostelium discoideum.Eukaryot Cell. 2004 Jun;3(3):646-62. doi: 10.1128/EC.3.3.646-662.2004. Eukaryot Cell. 2004. PMID: 15189986 Free PMC article.
-
Forty-five years of cGMP research in Dictyostelium: understanding the regulation and function of the cGMP pathway for cell movement and chemotaxis.Mol Biol Cell. 2021 Oct 1;32(20):ar8. doi: 10.1091/mbc.E21-04-0171. Epub 2021 Aug 4. Mol Biol Cell. 2021. PMID: 34347507 Free PMC article. Review.
-
cAMP signaling in Dictyostelium. Complexity of cAMP synthesis, degradation and detection.J Muscle Res Cell Motil. 2002;23(7-8):793-802. doi: 10.1023/a:1024483829878. J Muscle Res Cell Motil. 2002. PMID: 12952077 Review.
Cited by
-
Rab8a regulates the exocyst-mediated kiss-and-run discharge of the Dictyostelium contractile vacuole.Mol Biol Cell. 2012 Apr;23(7):1267-82. doi: 10.1091/mbc.E11-06-0576. Epub 2012 Feb 9. Mol Biol Cell. 2012. PMID: 22323285 Free PMC article.
-
Regulation of the Total Cell Surface Area in Dividing Dictyostelium Cells.Front Cell Dev Biol. 2020 Apr 8;8:238. doi: 10.3389/fcell.2020.00238. eCollection 2020. Front Cell Dev Biol. 2020. PMID: 32322581 Free PMC article.
-
The Polarized Redistribution of the Contractile Vacuole to the Rear of the Cell is Critical for Streaming and is Regulated by PI(4,5)P2-Mediated Exocytosis.Front Cell Dev Biol. 2022 Jul 19;9:765316. doi: 10.3389/fcell.2021.765316. eCollection 2021. Front Cell Dev Biol. 2022. PMID: 35928786 Free PMC article. Review.
-
The LRRK2-related Roco kinase Roco2 is regulated by Rab1A and controls the actin cytoskeleton.Mol Biol Cell. 2011 Jul 1;22(13):2198-211. doi: 10.1091/mbc.E10-12-0937. Epub 2011 May 5. Mol Biol Cell. 2011. PMID: 21551065 Free PMC article.
-
Bleb Expansion in Migrating Cells Depends on Supply of Membrane from Cell Surface Invaginations.Dev Cell. 2017 Dec 4;43(5):577-587.e5. doi: 10.1016/j.devcel.2017.10.030. Epub 2017 Nov 22. Dev Cell. 2017. PMID: 29173819 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

