The autophagic degradation of chloroplasts via rubisco-containing bodies is specifically linked to leaf carbon status but not nitrogen status in Arabidopsis
- PMID: 20807997
- PMCID: PMC2971599
- DOI: 10.1104/pp.110.158519
The autophagic degradation of chloroplasts via rubisco-containing bodies is specifically linked to leaf carbon status but not nitrogen status in Arabidopsis
Abstract
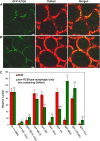
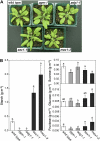
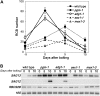
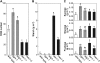
Autophagy is an intracellular process facilitating the vacuolar degradation of cytoplasmic components and is important for nutrient recycling during starvation. We previously demonstrated that chloroplasts can be partially mobilized to the vacuole by autophagy via spherical bodies named Rubisco-containing bodies (RCBs). Although chloroplasts contain approximately 80% of total leaf nitrogen and represent a major carbon and nitrogen source for new growth, the relationship between leaf nutrient status and RCB production remains unclear. We examined the effects of nutrient factors on the appearance of RCBs in leaves of transgenic Arabidopsis (Arabidopsis thaliana) expressing stroma-targeted fluorescent proteins. In excised leaves, the appearance of RCBs was suppressed by the presence of metabolic sugars, which were added externally or were produced during photosynthesis in the light. The light-mediated suppression was relieved by the inhibition of photosynthesis. During a diurnal cycle, RCB production was suppressed in leaves excised at the end of the day with high starch content. Starchless mutants phosphoglucomutase and ADP-Glc pyrophosphorylase1 produced a large number of RCBs, while starch-excess mutants starch-excess1 and maltose-excess1 produced fewer RCBs. In nitrogen-limited plants, as leaf carbohydrates were accumulated, RCB production was suppressed. We propose that there exists a close relationship between the degradation of chloroplast proteins via RCBs and leaf carbon but not nitrogen status in autophagy. We also found that the appearance of non-RCB-type autophagic bodies was not suppressed in the light and somewhat responded to nitrogen in excised leaves, unlike RCBs. These results imply that the degradation of chloroplast proteins via RCBs is specifically controlled in autophagy.
Figures






Similar articles
-
The changes of leaf carbohydrate contents as a regulator of autophagic degradation of chloroplasts via Rubisco-containing bodies during leaf senescence.Plant Signal Behav. 2011 May;6(5):685-7. doi: 10.4161/psb.6.5.14949. Epub 2011 May 1. Plant Signal Behav. 2011. PMID: 21499029 Free PMC article.
-
Mobilization of rubisco and stroma-localized fluorescent proteins of chloroplasts to the vacuole by an ATG gene-dependent autophagic process.Plant Physiol. 2008 Sep;148(1):142-55. doi: 10.1104/pp.108.122770. Epub 2008 Jul 9. Plant Physiol. 2008. PMID: 18614709 Free PMC article.
-
RCB-mediated chlorophagy caused by oversupply of nitrogen suppresses phosphate-starvation stress in plants.Plant Physiol. 2021 Mar 15;185(2):318-330. doi: 10.1093/plphys/kiaa030. Plant Physiol. 2021. PMID: 33721901 Free PMC article.
-
Roles of autophagy in chloroplast recycling.Biochim Biophys Acta. 2014 Apr;1837(4):512-21. doi: 10.1016/j.bbabio.2013.11.009. Epub 2013 Nov 19. Biochim Biophys Acta. 2014. PMID: 24269172 Review.
-
Leaf senescence and nutrient remobilisation in barley and wheat.Plant Biol (Stuttg). 2008 Sep;10 Suppl 1:37-49. doi: 10.1111/j.1438-8677.2008.00114.x. Plant Biol (Stuttg). 2008. PMID: 18721310 Review.
Cited by
-
Characterization of metabolic states of Arabidopsis thaliana under diverse carbon and nitrogen nutrient conditions via targeted metabolomic analysis.Plant Cell Physiol. 2014 Feb;55(2):306-19. doi: 10.1093/pcp/pct192. Epub 2013 Dec 15. Plant Cell Physiol. 2014. PMID: 24343996 Free PMC article.
-
Autophagy contributes to nighttime energy availability for growth in Arabidopsis.Plant Physiol. 2013 Apr;161(4):1682-93. doi: 10.1104/pp.113.215632. Epub 2013 Mar 1. Plant Physiol. 2013. PMID: 23457226 Free PMC article.
-
Establishment of monitoring methods for autophagy in rice reveals autophagic recycling of chloroplasts and root plastids during energy limitation.Plant Physiol. 2015 Apr;167(4):1307-20. doi: 10.1104/pp.114.254078. Epub 2015 Feb 25. Plant Physiol. 2015. PMID: 25717038 Free PMC article.
-
Autophagic degradation of membrane-bound organelles in plants.Biosci Rep. 2023 Jan 31;43(1):BSR20221204. doi: 10.1042/BSR20221204. Biosci Rep. 2023. PMID: 36562332 Free PMC article. Review.
-
Autophagy and Nutrients Management in Plants.Cells. 2019 Nov 12;8(11):1426. doi: 10.3390/cells8111426. Cells. 2019. PMID: 31726766 Free PMC article. Review.
References
-
- Acevedo-Hernández GJ, León P, Herrera-Estrella LR. (2005) Sugar and ABA responsiveness of a minimal RBCS light-responsive unit is mediated by direct binding of ABI4. Plant J 43: 506–519 - PubMed
-
- Aubert S, Gout E, Bligny R, Marty-Mazars D, Barrieu F, Alabouvette J, Marty F, Douce R. (1996) Ultrastructural and biochemical characterization of autophagy in higher plant cells subjected to carbon deprivation: control by the supply of mitochondria with respiratory substrates. J Cell Biol 133: 1251–1263 - PMC - PubMed
-
- Bassham DC. (2009) Function and regulation of macroautophagy in plants. Biochim Biophys Acta 1793: 1397–1403 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

