Comparison of Hybrid capture 2 testing at different thresholds with cytology as primary cervical screening test
- PMID: 20808310
- PMCID: PMC2965874
- DOI: 10.1038/sj.bjc.6605869
Comparison of Hybrid capture 2 testing at different thresholds with cytology as primary cervical screening test
Abstract
Background: We evaluated the performance of primary high-risk human papillomavirus (hrHPV) testing by hybrid capture 2 (HC2) with different thresholds for positivity, in comparison with conventional cytology.
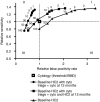
Methods: We used data of 25,871 women (aged 30-60 years) from the intervention group of the VUSA-Screen study (VU University Medical Center and Saltro laboratory population-based cervical screening study), who were screened by cytology and hrHPV. Primary outcome measure was the number of cervical intraepithelial neoplasia grade 3 or higher (CIN3+), detected within 3 years. We compared baseline cytology testing with three possible hrHPV screening strategies at different relative light unit/cutoff (RLU/CO) thresholds.
Results: Compared with baseline cytology testing, hrHPV DNA testing as a sole primary screening instrument did not yield a superior sensitivity, as well as lower colposcopy referral rate and lower false positivity rate at any RLU/CO threshold. The hrHPV screening at 1 RLU/CO threshold with cytology triage at baseline and at 12 months revealed the highest sensitivity for CIN3+ (relative sensitivity of 1.32), although still displaying a lower colposcopy referral rate than cytology testing (relative colposcopy rate of 0.94). Higher thresholds (>1 RLU/CO) yielded lower colposcopy rates, but resulted in substantial loss in sensitivity.
Conclusions: The hrHPV testing at the commonly used threshold of 1 RLU/CO with cytology triage at baseline and at 12 months showed a much higher sensitivity with a lower colposcopy referral rate compared with cytology testing.
Figures
References
-
- Anderson MC (1995) Premalignant and malignant squamous lesions of the cervix. In Haines and Taylor's: Obsterical and gynaecological pathology, Fox H, Wells M (eds), 4th edn, Chruchill Livingstone: New York, pp 292–297
-
- Arbyn M, Sasieni P, Meijer CJ, Clavel C, Koliopoulos G, Dillner J (2006) Chapter 9: clinical applications of HPV testing: a summary of meta-analyses. Vaccine 24(Suppl 3): S78–S89 - PubMed
-
- Begg CB, Greenes RA (1983) Assessment of diagnostic tests when disease verification is subject to selection bias. Biometrics 39: 207–215 - PubMed
-
- Berkhof J, Coupe VM, Bogaards JA, van Kemenade FJ, Helmerhorst TJ, Snijders PJ, Meijer CJ (2010) The health and economic effects of HPV DNA screening in The Netherlands. Int J Cancer, published online: 28 January 2010 - PubMed

