A phase I/II study of siltuximab (CNTO 328), an anti-interleukin-6 monoclonal antibody, in metastatic renal cell cancer
- PMID: 20808314
- PMCID: PMC2967052
- DOI: 10.1038/sj.bjc.6605872
A phase I/II study of siltuximab (CNTO 328), an anti-interleukin-6 monoclonal antibody, in metastatic renal cell cancer
Abstract
Background: Serum interleukin (IL)-6 levels correlate with disease outcomes in renal cell carcinoma (RCC) patients. Siltuximab, a chimeric, murine-human mAb against IL-6, was evaluated in a three-part phase I/II study in patients with progressive metastatic RCC.
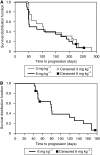
Methods: In part 1, 11 patients received 1, 3, 6, or 12mgkg-¹ at weeks 1, 4 and q2w × 2 thereafter; in part 2, 37 patients randomly received 3 or 6 mgkg-¹ q3w × 4; in part 3, 20 low-risk patients received 6mgkg-¹ q2w × 6. Modified WHO response criteria were assessed at weeks 7, 11, the 6-week follow-up, and when clinically indicated.
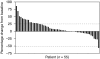
Results: Siltuximab was well tolerated overall, with no maximum tolerated dose or immune response observed. In all, 5 out of 11, 17 out of 37, and 9 out of 20 patients in parts 1, 2, and 3, respectively, received extended treatment beyond 4-6 initial infusions. In part 2, stable disease (SD) (≥11weeks) or better was achieved by 11 out of 17 (65%) 3 mgkg-¹ treated patients (one partial response (PR) ~8 months, 10 SD) and 10 out of 20 (50%) 6mgkg-¹ treated patients (10 SD). In part 3, documented complete or PR was not observed, but 13 out of 20 (65%) patients achieved SD.
Conclusion: Siltuximab stabilised disease in >50% of progressive metastatic RCC patients. One PR was observed. Given the favourable safety profile of siltuximab and poor correlation of tumour shrinkage with clinical benefit demonstrated for other non-cytotoxic therapies, further evaluation of dose-escalation strategies and/or combination therapy may be considered for patients with RCC.
Figures

Comment in
-
Is immunotherapy re-entering the kidney cancer arena from the back door? Considerations from the Phase I/II study of siltuximab.Immunotherapy. 2011 Apr;3(4):487-90. doi: 10.2217/imt.11.19. Immunotherapy. 2011. PMID: 21463189
References
-
- Altundag O, Altundag K, Gunduz E (2005) Interleukin-6 and C-reactive protein in metastatic renal cell carcinoma. J Clin Oncol 23: 1044; author reply 1044-1045 - PubMed
-
- Blay JY, Negrier S, Combaret V, Attali S, Goillot E, Merrouche Y, Mercatello A, Ravault A, Tourani JM, Moskovtchenko JF, Philip T, Favrot M (1992) Serum level of interleukin 6 as a prognosis factor in metastatic renal cell carcinoma. Cancer Res 52: 3317–3322 - PubMed
-
- Blay JY, Rossi JF, Wijdenes J, Menetrier-Caux C, Schemann S, Negrier S, Philip T, Favrot M (1997) Role of interleukin-6 in the paraneoplastic inflammatory syndrome associated with renal-cell carcinoma. Int J Cancer 72: 424–430 - PubMed
-
- Capuron L, Ravaud A, Gualde N, Bosmans E, Dantzer R, Maes M, Neveu PJ (2001) Association between immune activation and early depressive symptoms in cancer patients treated with interleukin-2-based therapy. Psychoneuroendocrinology 26: 797–808 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

