Context dependent benzodiazepine modulation of GABA(A) receptor opening frequency
- PMID: 20808542
- PMCID: PMC2866457
- DOI: 10.2174/157015910790909467
Context dependent benzodiazepine modulation of GABA(A) receptor opening frequency
Abstract
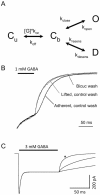
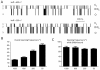
The anxiolytic, hypnotic, and anti-convulsant properties of benzodiazepines (BDZs) require modulation of distinct GABA(A) receptor alpha-subtypes. BDZ modulation of GABA(A) receptors is often described in terms of increased opening frequency, and contrasted with the increased open durations occurring with barbiturate modulation. Several studies spanning single channel, rapid kinetic, and whole cell techniques have suggested that BDZs effect this observed change in frequency through increased affinity for GABA. BDZ-sensitive αβγ isoforms exist at extrasynaptic as well as synaptic locations, where they encounter markedly different concentration and time-course of GABA exposure. Interestingly, this affinity-based mechanism (specifically, decreasing the GABA unbinding rate) is only predicted to increase opening frequency under conditions that allow the unbinding and rebinding cycles typical of prolonged exposure to low GABA concentrations, which are more likely to occur at extrasynaptic GABA(A) receptors. In contrast, when rebinding is less likely, such as may occur in certain synaptic conditions, the number, but not the frequency, of channel openings increases in response to BDZ modulation. In conclusion, not only can multiple kinetic mechanisms alter channel opening frequency, but a single mechanism - increased affinity - impacts opening frequency differently under different contexts of GABA(A) receptor activation.
Keywords: Mechanism; affinity.; extra-synaptic; ion channel; synaptic.
Figures


References
-
- Bianchi MT, Macdonald RL. Mutation of the 9' leucine in the GABA(A) receptor gamma2L subunit produces an apparent decrease in desensitization by stabilizing open states without altering desensitized states. Neuropharmacology. 2001;41(6):737–44. - PubMed
LinkOut - more resources
Full Text Sources
