Pegfilgrastim on the Same Day Versus Next Day of Chemotherapy in Patients With Breast Cancer, Non-Small-Cell Lung Cancer, Ovarian Cancer, and Non-Hodgkin's Lymphoma: Results of Four Multicenter, Double-Blind, Randomized Phase II Studies
- PMID: 20808556
- PMCID: PMC2868638
- DOI: 10.1200/JOP.091094
Pegfilgrastim on the Same Day Versus Next Day of Chemotherapy in Patients With Breast Cancer, Non-Small-Cell Lung Cancer, Ovarian Cancer, and Non-Hodgkin's Lymphoma: Results of Four Multicenter, Double-Blind, Randomized Phase II Studies
Abstract
Purpose: To compare data on severe (grade 4) neutropenia duration and febrile neutropenia incidence in patients receiving chemotherapy with pegfilgrastim administered the same day or 24 hours after chemotherapy.
Patients and methods: These were similar, randomized, double-blind phase II noninferiority studies of patients with lymphoma or non-small-cell lung (NSCLC), breast, or ovarian cancer. Each study was analyzed separately. The primary end point in each study was cycle-1 severe neutropenia duration. Approximately 90 patients per study were to be randomly assigned at a ratio of 1:1 to receive pegfilgrastim 6 mg once per cycle on the day of chemotherapy or the day after (with placebo on the alternate day).
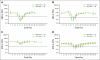
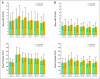
Results: In four studies, 272 patients received chemotherapy and one or more doses of pegfilgrastim (133 same day, 139 next day). Three studies (breast, lymphoma, NSCLC) enrolled an adequate number of patients for analysis. However, in the NSCLC study, the neutropenic rate was lower than expected (only two patients per arm experienced grade 4 neutropenia). In the breast cancer study, the mean cycle-1 severe neutropenia duration was 1.2 days (95% confidence limit [CL], 0.7 to 1.6) longer in the same-day compared with the next-day group (mean, 2.6 v 1.4 days). In the lymphoma study, the mean cycle-1 severe neutropenia duration was 0.9 days (95% CL, 0.3 to 1.4) longer in the same-day compared with the next-day group (mean, 2.1 v 1.2 days). In the breast and lymphoma studies, the absolute neutrophil count profile for same-day patients was earlier, deeper, and longer compared with that for next-day patients, although the results indicate that same-day administration was statistically noninferior to next-day administration according to neutropenia duration.
Conclusion: For patients receiving pegfilgrastim with chemotherapy, pegfilgrastim administered 24 hours after chemotherapy completion is recommended.
Figures
References
-
- Kuderer NM, Dale DC, Crawford J, et al. Mortality, morbidity, and cost associated with febrile neutropenia in adult cancer patients. Cancer. 2006;106:2258–2266. - PubMed
-
- Link BK, Budd GT, Scott S, et al. Delivering adjuvant chemotherapy to women with early-stage breast carcinoma: Current patterns of care. Cancer. 2001;92:1354–1367. - PubMed
-
- Crawford J, Ozer H, Stoller R, et al. Reduction by granulocyte colony-stimulating factor of fever and neutropenia induced by chemotherapy in patients with small-cell lung cancer. N Engl J Med. 1991;325:164–170. - PubMed
-
- Trillet-Lenoir V, Green J, Manegold C, et al. Recombinant granulocyte colony stimulating factor reduces the infectious complications of cytotoxic chemotherapy. Eur J Cancer. 1993;29A:319–324. - PubMed
-
- Molineux G, Dexter TM. Biology of G-CSF. In: Morstyn G, Dexter TM, Foote M, editors. Filgrastim (r-metHuG-CSF) in Clinical Practice. ed 2. New York, NY: Informa Healthcare; 1998. pp. 51–71.pp. 606–607.
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

