Aldosterone modulates cell proliferation and apoptosis in the neonatal rat heart
- PMID: 20808672
- PMCID: PMC2923799
- DOI: 10.3346/jkms.2010.25.9.1296
Aldosterone modulates cell proliferation and apoptosis in the neonatal rat heart
Abstract
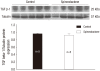
In the present study, we investigated whether and how the mineralocorticoid receptor antagonist spironolactone affects cardiac growth and development through apoptosis and cell proliferation in the neonatal rat heart. Newborn rat pups were treated with spironolactone (200 mg/kg/d) for 7 days. The cell proliferation was studied by PCNA immunostaining. The treatment with spironolactone decreased proliferating myocytes by 32% (P<0.05), and reduced myocytes apoptosis by 29% (P<0.05). Immunoblot and immunohistochemistry for the expression of p38, p53, clusterin, TGF-beta2, and extracellular signal-regulated kinase were performed. In the spironolactone group, p38, p53, clusterin, and TGF-beta2 protein expression was significantly decreased (P<0.05). These results indicate that aldosterone inhibition in the developing rat heart induces cardiac growth impairment by decreasing proliferation and apoptosis of myocytes.
Keywords: Aldosterone; Apoptosis; Cell Proliferation; Muscle Cells.
Figures


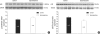
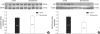
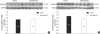
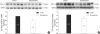
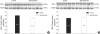

Similar articles
-
Angiotensin converting enzyme inhibition decreases cell turnover in the neonatal rat heart.Pediatr Res. 2002 Sep;52(3):325-32. doi: 10.1203/00006450-200209000-00004. Pediatr Res. 2002. PMID: 12193663
-
Fenofibrate inhibits aldosterone-induced apoptosis in adult rat ventricular myocytes via stress-activated kinase-dependent mechanisms.Am J Physiol Heart Circ Physiol. 2009 Jun;296(6):H1983-93. doi: 10.1152/ajpheart.00002.2009. Epub 2009 Apr 24. Am J Physiol Heart Circ Physiol. 2009. PMID: 19395558 Free PMC article.
-
Aldosterone regulates cellular turnover and mitogen-activated protein kinase family expression in the neonatal rat kidney.J Cell Physiol. 2009 Jun;219(3):724-33. doi: 10.1002/jcp.21723. J Cell Physiol. 2009. PMID: 19202554
-
Mineralocorticoid receptor antagonists: the evolution of utility and pharmacology.Kidney Int. 2000 Apr;57(4):1408-11. doi: 10.1046/j.1523-1755.2000.00983.x. Kidney Int. 2000. PMID: 10760075 Review.
-
Pleiotropic actions of aldosterone and the effects of eplerenone, a selective mineralocorticoid receptor antagonist.Hypertens Res. 2004 Nov;27(11):781-9. doi: 10.1291/hypres.27.781. Hypertens Res. 2004. PMID: 15824460 Review.
Cited by
-
Comparison of stress-induced changes in adults and pups: is aldosterone the main adrenocortical stress hormone during the perinatal period in rats?PLoS One. 2013 Sep 5;8(9):e72313. doi: 10.1371/journal.pone.0072313. eCollection 2013. PLoS One. 2013. PMID: 24039750 Free PMC article.
-
Cortisol stimulates proliferation and apoptosis in the late gestation fetal heart: differential effects of mineralocorticoid and glucocorticoid receptors.Am J Physiol Regul Integr Comp Physiol. 2013 Aug 15;305(4):R343-50. doi: 10.1152/ajpregu.00112.2013. Epub 2013 Jun 19. Am J Physiol Regul Integr Comp Physiol. 2013. PMID: 23785077 Free PMC article.
-
Aldosterone deficiency adversely affects pregnancy outcome in mice.Pflugers Arch. 2012 Oct;464(4):331-43. doi: 10.1007/s00424-012-1145-4. Epub 2012 Sep 2. Pflugers Arch. 2012. PMID: 22941338
-
Glucocorticoid receptor alters isovolumetric contraction and restrains cardiac fibrosis.J Endocrinol. 2017 Mar;232(3):437-450. doi: 10.1530/JOE-16-0458. Epub 2017 Jan 5. J Endocrinol. 2017. PMID: 28057868 Free PMC article.
-
Hypertension and cellular senescence.Biogerontology. 2023 Aug;24(4):457-478. doi: 10.1007/s10522-023-10031-4. Epub 2023 Apr 3. Biogerontology. 2023. PMID: 37010665 Review.
References
-
- Choi JH, Yoo KH, Cheon HW, Kim KB, Hong YS, Lee JW, Kim SK, Kim CH. Angiotensin converting enzyme inhibition decreases cell turnover in the neonatal rat heart. Pediatr Res. 2002;52:325–332. - PubMed
-
- Burniston JG, Saini A, Tan LB, Goldspink DF. Aldosterone induces myocyte apoptosis in the heart and skeletal muscles of rats in vivo. J Mol Cell Cardiol. 2005;39:395–399. - PubMed
-
- Veliotes DG, Woodiwiss A, Deftereos DA, Gray D, Osadchii O, Norton GR. Aldosterone receptor blockade prevents the transition to cardiac pump dysfunction induced by beta-adrenoreceptor activation. Hypertension. 2005;45:914–920. - PubMed
-
- Wang W, McClain JM, Zucker IH. Aldosterone reduces baroreceptor discharge in the dog. Hypertension. 1992;19:270–277. - PubMed
-
- Connell JM, Davies E. The new biology of aldosterone. J Endocrinol. 2005;186:1–20. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

