Expression changes in DNA repair enzymes and mitochondrial DNA damage in aging rat lens
- PMID: 20808729
- PMCID: PMC2929939
Expression changes in DNA repair enzymes and mitochondrial DNA damage in aging rat lens
Abstract
Purpose: To determine if there is increased mitochondrial DNA (mtDNA) and nuclear DNA (nDNA) damage with age in the lenses of rats. We also explored the immunolocalization of 8-oxoguanine DNA glycosylase 1 (OGG1) and AP endonuclease 1 (APE1) in the lens and studied three of the predominant base excision repair (BER) enzymes: OGG1, APE1, and DNA polymerase gamma (Polgamma).
Methods: The methods used by this study include the selection of twenty-six male Wistar rats in each group (2 months old and 26 months old) and fourteen male Wistar rats in the 16 months old group. The total DNA of lenses were isolated and the DNA genome was amplified by a long extension-polymerase chain reaction (LX-PCR). We examined mtDNA and nDNA damage with a quantitative polymerase chain reaction (QPCR) assay that was combined with EvaGreen. We also studied the gene expression of mRNA and protein in these key BER enzymes with real time-polymerase chain reaction (RT-PCR) and western blot analysis.
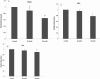
Results: There was an increase in oxidative DNA damage, which exists primarily in the mtDNA. The amount of 8-hydroxy-2'-deoxy-guanosine (8-OHdG) in DNA was significantly increased with age. Our experiments demonstrated that the gene expression of mRNA and protein in these key BER enzymes decreased with age. OGG1 and APE1 were localized by immunohistochemistry within lens epithelial cells (LECs) and superficial fiber cells.
Conclusions: The gene expression of mRNA and protein in these key BER enzymes decreased with age, which caused a decrease in the repairing capability of the mtDNA and the accumulation of mtDNA damage. The increased mtDNA damage and decreased expression of BER enzymes may cause a "vicious cycle" of oxidative stress that contributes to the accumulation of mtDNA mutations and age-related cataract pathogenesis.
Figures
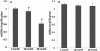
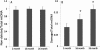
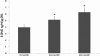


Similar articles
-
Increased mitochondrial DNA damage and down-regulation of DNA repair enzymes in aged rodent retinal pigment epithelium and choroid.Mol Vis. 2008 Apr 4;14:644-51. Mol Vis. 2008. PMID: 18392142 Free PMC article.
-
The changes of 8-OHdG, hOGG1, APE1 and Pol β in lenses of patients with age-related cataract.Curr Eye Res. 2015 Apr;40(4):378-85. doi: 10.3109/02713683.2014.924148. Epub 2014 Jun 9. Curr Eye Res. 2015. PMID: 24911554
-
Oxygen-induced changes in mitochondrial DNA and DNA repair enzymes in aging rat lens.Mech Ageing Dev. 2010 Nov-Dec;131(11-12):666-73. doi: 10.1016/j.mad.2010.09.003. Epub 2010 Sep 18. Mech Ageing Dev. 2010. PMID: 20854836
-
Mitochondrial base excision repair assays.Methods. 2010 Aug;51(4):416-25. doi: 10.1016/j.ymeth.2010.02.020. Epub 2010 Feb 25. Methods. 2010. PMID: 20188838 Free PMC article. Review.
-
Mitochondrial repair of 8-oxoguanine and changes with aging.Exp Gerontol. 2002 Oct-Nov;37(10-11):1189-96. doi: 10.1016/s0531-5565(02)00142-0. Exp Gerontol. 2002. PMID: 12470830 Review.
Cited by
-
β-hydroxybutyrate recapitulates the beneficial effects of ketogenic metabolic therapy in polycystic kidney disease.iScience. 2024 Aug 20;27(9):110773. doi: 10.1016/j.isci.2024.110773. eCollection 2024 Sep 20. iScience. 2024. PMID: 39314240 Free PMC article.
-
In vivo and in vitro studies evaluating the chemopreventive effect of metformin on the aryl hydrocarbon receptor-mediated breast carcinogenesis.Saudi J Biol Sci. 2021 Dec;28(12):7396-7403. doi: 10.1016/j.sjbs.2021.08.051. Epub 2021 Aug 27. Saudi J Biol Sci. 2021. PMID: 34867043 Free PMC article.
-
Association between the 8-oxoguanine DNA glycosylase gene Ser326Cys polymorphism and age-related cataract: a systematic review and meta-analysis.Int Ophthalmol. 2018 Aug;38(4):1451-1457. doi: 10.1007/s10792-017-0606-3. Epub 2017 Jun 19. Int Ophthalmol. 2018. PMID: 28631182
-
Mitochondrial stress signaling promotes cellular adaptations.Int J Cell Biol. 2014;2014:156020. doi: 10.1155/2014/156020. Epub 2014 Jan 22. Int J Cell Biol. 2014. PMID: 24587804 Free PMC article. Review.
-
A combination of β-hydroxybutyrate and citrate ameliorates disease progression in a rat model of polycystic kidney disease.Am J Physiol Renal Physiol. 2024 Mar 1;326(3):F352-F368. doi: 10.1152/ajprenal.00205.2023. Epub 2023 Dec 14. Am J Physiol Renal Physiol. 2024. PMID: 38095025 Free PMC article.
References
-
- Ozmen B, Ozmen D, Erkin E. Lens superoxide dismutase and catalase activeties in diabetic cataract. Clin Biochem. 2002;35:69–72. - PubMed
-
- Bantseev VL, Herbert T, Trevithick JR, Sivak JG. Mitochondria of rat lenses: Distribution near and at the sutures. Curr Eye Res. 1999;19:506–16. - PubMed
-
- Bantseev V, McCanna D, Banh A, Wong WW, Moran KL, Dixon DG. Mechanisms of ocular toxicity using the in vitro bovine lens and sodium dodecyl sulfate as a chemical model. Toxicol Sci. 2003;73:98–107. - PubMed
-
- Huang L, Yappert MC, Jumblatt MM, Borchman D. Hyperoxia and thyroxine treatment and the relationships between reactive oxygen species generation, mitochondrial membrane potential and cardiolipin in human lens epithelial cell cultures. Curr Eye Res. 2008;33:575–86. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous
