Tyrosine-phosphorylated caveolin-1 blocks bacterial uptake by inducing Vav2-RhoA-mediated cytoskeletal rearrangements
- PMID: 20808760
- PMCID: PMC2927421
- DOI: 10.1371/journal.pbio.1000457
Tyrosine-phosphorylated caveolin-1 blocks bacterial uptake by inducing Vav2-RhoA-mediated cytoskeletal rearrangements
Abstract
Certain bacterial adhesins appear to promote a pathogen's extracellular lifestyle rather than its entry into host cells. However, little is known about the stimuli elicited upon such pathogen host-cell interactions. Here, we report that type IV pili (Tfp)-producing Neisseria gonorrhoeae (P(+)GC) induces an immediate recruitment of caveolin-1 (Cav1) in the host cell, which subsequently prevents bacterial internalization by triggering cytoskeletal rearrangements via downstream phosphotyrosine signaling. A broad and unbiased analysis of potential interaction partners for tyrosine-phosphorylated Cav1 revealed a direct interaction with the Rho-family guanine nucleotide exchange factor Vav2. Both Vav2 and its substrate, the small GTPase RhoA, were found to play a direct role in the Cav1-mediated prevention of bacterial uptake. Our findings, which have been extended to enteropathogenic Escherichia coli, highlight how Tfp-producing bacteria avoid host cell uptake. Further, our data establish a mechanistic link between Cav1 phosphorylation and pathogen-induced cytoskeleton reorganization and advance our understanding of caveolin function.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
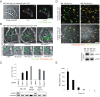



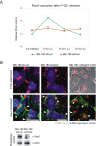
References
-
- Gerbase A. C, Rowley J. T, Mertens T. E. Global epidemiology of sexually transmitted diseases. Lancet. 1998;351(Suppl 3):2–4. - PubMed
-
- Merz A. J, So M. Interactions of pathogenic neisseriae with epithelial cell membranes. Annu Rev Cell Dev Biol. 2000;16:423–457. - PubMed
-
- Strom M. S, Lory S. Structure-function and biogenesis of the type IV pili. Annu Rev Microbiol. 1993;47:565–596. - PubMed
-
- Kline K. A, Falker S, Dahlberg S, Normark S, Henriques-Normark B. Bacterial adhesins in host-microbe interactions. Cell Host Microbe. 2009;5:580–592. - PubMed
-
- Mikaty G, Soyer M, Mairey E, Henry N, Dyer D, et al. Extracellular bacterial pathogen induces host cell surface reorganization to resist shear stress. PLoS Pathog. 2009;5:e1000314. doi: 10.1371/journal.ppat.1000314. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

