Endothelial membrane remodeling is obligate for anti-angiogenic radiosensitization during tumor radiosurgery
- PMID: 20808818
- PMCID: PMC2924400
- DOI: 10.1371/journal.pone.0012310
Endothelial membrane remodeling is obligate for anti-angiogenic radiosensitization during tumor radiosurgery
Erratum in
-
Endothelial membrane remodeling is obligate for anti-angiogenic radiosensitization during tumor radiosurgery.PLoS One. 2010 Sep 30;5(9):10.1371/annotation/6e222ad5-b175-4a00-9d04-4d120568a897. doi: 10.1371/annotation/6e222ad5-b175-4a00-9d04-4d120568a897. PLoS One. 2010. PMID: 20941382 Free PMC article.
Abstract
Background: While there is significant interest in combining anti-angiogenesis therapy with conventional anti-cancer treatment, clinical trials have as of yet yielded limited therapeutic gain, mainly because mechanisms of anti-angiogenic therapy remain to a large extent unknown. Currently, anti-angiogenic tumor therapy is conceptualized to either "normalize" dysfunctional tumor vasculature, or to prevent recruitment of circulating endothelial precursors into the tumor. An alternative biology, restricted to delivery of anti-angiogenics immediately prior to single dose radiotherapy (radiosurgery), is provided in the present study.
Methodology/principal findings: Genetic data indicate an acute wave of ceramide-mediated endothelial apoptosis, initiated by acid sphingomyelinase (ASMase), regulates tumor stem cell response to single dose radiotherapy, obligatory for tumor cure. Here we show VEGF prevented radiation-induced ASMase activation in cultured endothelium, occurring within minutes after radiation exposure, consequently repressing apoptosis, an event reversible with exogenous C(16)-ceramide. Anti-VEGFR2 acts conversely, enhancing ceramide generation and apoptosis. In vivo, MCA/129 fibrosarcoma tumors were implanted in asmase(+/+) mice or asmase(-/-) littermates and irradiated in the presence or absence of anti-VEGFR2 DC101 or anti-VEGF G6-31 antibodies. These anti-angiogenic agents, only if delivered immediately prior to single dose radiotherapy, de-repressed radiation-induced ASMase activation, synergistically increasing the endothelial apoptotic component of tumor response and tumor cure. Anti-angiogenic radiosensitization was abrogated in tumors implanted in asmase(-/-) mice that provide apoptosis-resistant vasculature, or in wild-type littermates pre-treated with anti-ceramide antibody, indicating that ceramide is necessary for this effect.
Conclusions/significance: These studies show that angiogenic factors fail to suppress apoptosis if ceramide remains elevated while anti-angiogenic therapies fail without ceramide elevation, defining a ceramide rheostat that determines outcome of single dose radiotherapy. Understanding the temporal sequencing of anti-angiogenic drugs and radiation enables optimized radiosensitization and design of innovative radiosurgery clinical trials.
Conflict of interest statement
Figures
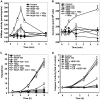
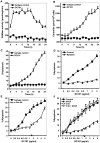
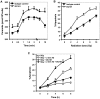

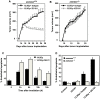

Similar articles
-
Endothelial membrane remodeling is obligate for anti-angiogenic radiosensitization during tumor radiosurgery.PLoS One. 2010 Sep 30;5(9):10.1371/annotation/6e222ad5-b175-4a00-9d04-4d120568a897. doi: 10.1371/annotation/6e222ad5-b175-4a00-9d04-4d120568a897. PLoS One. 2010. PMID: 20941382 Free PMC article.
-
Enhancement of Soft Tissue Sarcoma Response to Gemcitabine through Timed Administration of a Short-Acting Anti-Angiogenic Agent.Cell Physiol Biochem. 2020 Jul 29;54(4):707-718. doi: 10.33594/000000250. Cell Physiol Biochem. 2020. PMID: 32722909 Free PMC article.
-
Axitinib sensitization of high Single Dose Radiotherapy.Radiother Oncol. 2014 Apr;111(1):88-93. doi: 10.1016/j.radonc.2014.02.010. Epub 2014 Apr 29. Radiother Oncol. 2014. PMID: 24794795 Free PMC article.
-
The acid sphingomyelinase/ceramide pathway: biomedical significance and mechanisms of regulation.Curr Mol Med. 2010 Jul;10(5):454-66. doi: 10.2174/156652410791608225. Curr Mol Med. 2010. PMID: 20540705 Review.
-
Targeting the ceramide system in cancer.Cancer Lett. 2013 May 28;332(2):286-94. doi: 10.1016/j.canlet.2011.07.010. Epub 2011 Jul 23. Cancer Lett. 2013. PMID: 21862212 Review.
Cited by
-
Efficacy of BRAF Inhibitors in Combination With Stereotactic Radiosurgery for the Treatment of Melanoma Brain Metastases: A Systematic Review and Meta-Analysis.Front Oncol. 2021 Feb 22;10:586029. doi: 10.3389/fonc.2020.586029. eCollection 2020. Front Oncol. 2021. PMID: 33692938 Free PMC article.
-
Radiotherapy as a tool to elicit clinically actionable signalling pathways in cancer.Nat Rev Clin Oncol. 2022 Feb;19(2):114-131. doi: 10.1038/s41571-021-00579-w. Epub 2021 Nov 24. Nat Rev Clin Oncol. 2022. PMID: 34819622 Free PMC article. Review.
-
Factors that determine local control with gamma knife radiosurgery: The role of primary histology.J Radiosurg SBRT. 2015;3(4):281-286. J Radiosurg SBRT. 2015. PMID: 26478823 Free PMC article.
-
Results of a phase II trial of gemcitabine plus doxorubicin in patients with recurrent head and neck cancers: serum C₁₈-ceramide as a novel biomarker for monitoring response.Clin Cancer Res. 2011 Sep 15;17(18):6097-105. doi: 10.1158/1078-0432.CCR-11-0930. Epub 2011 Jul 26. Clin Cancer Res. 2011. PMID: 21791630 Free PMC article. Clinical Trial.
-
Radiosensitizing the SUMO stress response intensifies single-dose radiotherapy tumor cure.JCI Insight. 2025 May 22;10(12):e153601. doi: 10.1172/jci.insight.153601. eCollection 2025 Jun 23. JCI Insight. 2025. PMID: 40402574 Free PMC article.
References
-
- Sessa C, Guibal A, Del Conte G, Ruegg C. Biomarkers of angiogenesis for the development of antiangiogenic therapies in oncology: tools or decorations? Nat Clin Pract Oncol. 2008;5:378–391. - PubMed
-
- Folkman J. Tumor angiogenesis: therapeutic implications. N Engl J Med. 1971;285:1182–1186. - PubMed
-
- Paris F, Fuks Z, Kang A, Capodieci P, Juan G, et al. Endothelial apoptosis as the primary lesion initiating intestinal radiation damage in mice. Science. 2001;293:293–297. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

