Calcium-activated potassium channels BK and IK1 are functionally expressed in human gliomas but do not regulate cell proliferation
- PMID: 20808839
- PMCID: PMC2924897
- DOI: 10.1371/journal.pone.0012304
Calcium-activated potassium channels BK and IK1 are functionally expressed in human gliomas but do not regulate cell proliferation
Abstract
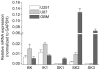
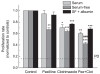
Gliomas are morbid brain tumors that are extremely resistant to available chemotherapy and radiology treatments. Some studies have suggested that calcium-activated potassium channels contribute to the high proliferative potential of tumor cells, including gliomas. However, other publications demonstrated no role for these channels or even assigned them antitumorogenic properties. In this work we characterized the expression and functional contribution to proliferation of Ca(2+)-activated K(+) channels in human glioblastoma cells. Quantitative RT-PCR detected transcripts for the big conductance (BK), intermediate conductance (IK1), and small conductance (SK2) K(+) channels in two glioblastoma-derived cell lines and a surgical sample of glioblastoma multiforme. Functional expression of BK and IK1 in U251 and U87 glioma cell lines and primary glioma cultures was verified using whole-cell electrophysiological recordings. Inhibitors of BK (paxilline and penitrem A) and IK1 channels (clotrimazole and TRAM-34) reduced U251 and U87 proliferation in an additive fashion, while the selective blocker of SK channels UCL1848 had no effect. However, the antiproliferative properties of BK and IK1 inhibitors were seen at concentrations that were higher than those necessary to inhibit channel activity. To verify specificity of pharmacological agents, we downregulated BK and IK1 channels in U251 cells using gene-specific siRNAs. Although siRNA knockdowns caused strong reductions in the BK and IK1 current densities, neither single nor double gene silencing significantly affected rates of proliferation. Taken together, these results suggest that Ca(2+)-activated K(+) channels do not play a critical role in proliferation of glioma cells and that the effects of pharmacological inhibitors occur through their off-target actions.
Conflict of interest statement
Figures





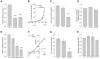
Similar articles
-
Differential role of IK and BK potassium channels as mediators of intrinsic and extrinsic apoptotic cell death.Am J Physiol Cell Physiol. 2012 Nov 15;303(10):C1070-8. doi: 10.1152/ajpcell.00040.2012. Epub 2012 Sep 19. Am J Physiol Cell Physiol. 2012. PMID: 22992678 Free PMC article.
-
Dynamic coupling between TRPV4 and Ca2+-activated SK1/3 and IK1 K+ channels plays a critical role in regulating the K+-secretory BK channel in kidney collecting duct cells.Am J Physiol Renal Physiol. 2017 Jun 1;312(6):F1081-F1089. doi: 10.1152/ajprenal.00037.2017. Epub 2017 Mar 8. Am J Physiol Renal Physiol. 2017. PMID: 28274924 Free PMC article.
-
Ca2+-activated K channels in parotid acinar cells: The functional basis for the hyperpolarized activation of BK channels.Channels (Austin). 2010 Jul-Aug;4(4):278-88. doi: 10.4161/chan.4.4.12197. Epub 2010 Jul 28. Channels (Austin). 2010. PMID: 20519930 Free PMC article.
-
Endothelium as target for large-conductance calcium-activated potassium channel openers.Acta Biochim Pol. 2009;56(3):393-404. Epub 2009 Sep 14. Acta Biochim Pol. 2009. PMID: 19753330 Review.
-
Airway Hydration, Apical K(+) Secretion, and the Large-Conductance, Ca(2+)-activated and Voltage-dependent Potassium (BK) Channel.Ann Am Thorac Soc. 2016 Apr;13 Suppl 2(Suppl 2):S163-8. doi: 10.1513/AnnalsATS.201507-405KV. Ann Am Thorac Soc. 2016. PMID: 27115952 Free PMC article. Review.
Cited by
-
Nodulisporic acid E biosynthesis: in vivo characterisation of NodD1, an indole-diterpene prenyltransferase that acts on an emindole SB derived indole-diterpene scaffold.Medchemcomm. 2019 May 27;10(7):1160-1164. doi: 10.1039/c9md00143c. eCollection 2019 Jul 1. Medchemcomm. 2019. PMID: 31391888 Free PMC article.
-
Potassium channels in cell cycle and cell proliferation.Philos Trans R Soc Lond B Biol Sci. 2014 Feb 3;369(1638):20130094. doi: 10.1098/rstb.2013.0094. Print 2014 Mar 19. Philos Trans R Soc Lond B Biol Sci. 2014. PMID: 24493742 Free PMC article. Review.
-
Downregulation of Leucine-Rich Repeat-Containing 8A Limits Proliferation and Increases Sensitivity of Glioblastoma to Temozolomide and Carmustine.Front Oncol. 2018 May 7;8:142. doi: 10.3389/fonc.2018.00142. eCollection 2018. Front Oncol. 2018. PMID: 29868469 Free PMC article.
-
Involvement of large-conductance Ca2+-activated K+ channels in chloroquine-induced force alterations in pre-contracted airway smooth muscle.PLoS One. 2015 Mar 30;10(3):e0121566. doi: 10.1371/journal.pone.0121566. eCollection 2015. PLoS One. 2015. PMID: 25822280 Free PMC article.
-
The large conductance Ca(2+) -activated K(+) (BKCa) channel regulates cell proliferation in SH-SY5Y neuroblastoma cells by activating the staurosporine-sensitive protein kinases.Front Physiol. 2014 Dec 9;5:476. doi: 10.3389/fphys.2014.00476. eCollection 2014. Front Physiol. 2014. PMID: 25538629 Free PMC article.
References
-
- Schwartzbaum JA, Fisher JL, Aldape KD, Wrensch M. Epidemiology and molecular pathology of glioma. Nat Clin Pract Neurol. 2006;2:494–503. - PubMed
-
- Kleihues P, Burger PC, Scheithauer BW. The new WHO classification of brain tumours. Brain Pathol. 1993;3:255–268. - PubMed
-
- Barnholtz-Sloan JS, Sloan AE, Schwartz AG. Relative survival rates and patterns of diagnosis analyzed by time period for individuals with primary malignant brain tumor, 1973-1997. J Neurosurg. 2003;99:458–466. - PubMed
-
- Sanai N, Berger MS. Glioma extent of resection and its impact on patient outcome. Neurosurgery. 2008;62:753–764. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

